Open Question
What is the oxidation state of the group 3A element in each of the following compounds? (a) NaBF4 (b) GaCl3 (c) TlCl (d) B2H6
 Verified step by step guidance
Verified step by step guidance

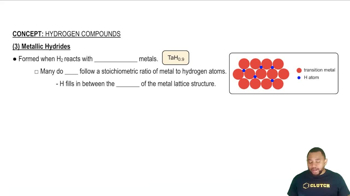

(a) Describe what is meant by an electron-deficient molecule.
(b) Describe what is meant by a three-center, two-electron bond.
Identify the group 4A element that best fits each of the following descriptions.
(b) Forms the strongest π bonds
Identify the group 4A element that best fits each of the following descriptions.
(c) Is the second most abundant element in the Earth's crust
Select the group 4A element that best fits each of the following descriptions.
(c) Is the second most abundant element in the human body