This reaction was monitored as a function of time: AB → A + B A plot of 1/[AB] versus time yields a straight line with a slope of +0.55/Ms. b. Write the rate law for the reaction.
a. What is the half-life for the first-order decomposition of SO2Cl2 with a rate constant of 1.42 x 10^-4 s^-1? b. How long will it take for the concentration of SO2Cl2 to decrease to 25% of its initial concentration? c. If the initial concentration of SO2Cl2 is 1.00 M, how long will it take for the concentration to decrease to 0.78 M? d. If the initial concentration of SO2Cl2 is 0.150 M, what is the concentration of SO2Cl2 after 2.00 x 10^2 s? After 5.00 x 10^2 s?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
First-Order Reactions

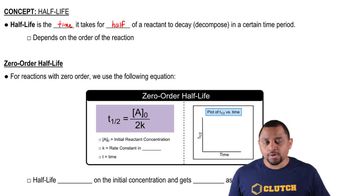
Half-Life
Exponential Decay

This reaction was monitored as a function of time: AB → A + B A plot of 1/[AB] versus time yields a straight line with a slope of +0.55/Ms. c. What is the half-life when the initial concentration is 0.55 M?
This reaction was monitored as a function of time: AB → A + B A plot of 1/[AB] versus time yields a straight line with a slope of +0.55/Ms.
d. If the initial concentration of AB is 0.250 M, and the reaction mixture initially contains no products, what are the concentrations of A and B after 75 s?
The decomposition of XY is second order in XY and has a rate constant of 7.02⨉10-3 M-1• s-1 at a certain temperature. a. What is the half-life for this reaction at an initial concentration of 0.100 M?
The half-life for the radioactive decay of U-238 is 4.5 billion years and is independent of initial concentration. How long will it take for 10% of the U-238 atoms in a sample of U-238 to decay?
