Textbook Question
How many hydrogen atoms are in each of the following: (a) C2H5OH? (b) Ca(C2H5COO)2? (c) (NH4)3PO4?
547
views

 Verified step by step guidance
Verified step by step guidance

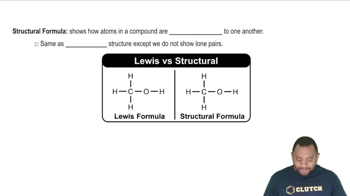

How many hydrogen atoms are in each of the following: (a) C2H5OH? (b) Ca(C2H5COO)2? (c) (NH4)3PO4?
How many of the indicated atoms are represented by each chemical formula: (a) carbon atoms in C4H9COOCH3
How many of the indicated atoms are represented by each chemical formula: (b) oxygen atoms in Ca(ClO3)2 (c) hydrogen atoms in (NH4)2HPO4?
Write the molecular and structural formulas for the compounds represented by the following models:
Fill in the gaps in the following table: Symbol 59Co3+ Protons 34 76 80 Neutrons 46 116 120 Electrons 36 78 Net charge 2+
Fill in the gaps in the following table: Symbol 133Cs+ Protons 35 15 Neutrons 46 16 30 Electrons 18 20 Net charge 1- 5+