Table of contents
- 0. Functions7h 52m
- Introduction to Functions16m
- Piecewise Functions10m
- Properties of Functions9m
- Common Functions1h 8m
- Transformations5m
- Combining Functions27m
- Exponent rules32m
- Exponential Functions28m
- Logarithmic Functions24m
- Properties of Logarithms34m
- Exponential & Logarithmic Equations35m
- Introduction to Trigonometric Functions38m
- Graphs of Trigonometric Functions44m
- Trigonometric Identities47m
- Inverse Trigonometric Functions48m
- 1. Limits and Continuity2h 2m
- 2. Intro to Derivatives1h 33m
- 3. Techniques of Differentiation3h 18m
- 4. Applications of Derivatives2h 38m
- 5. Graphical Applications of Derivatives6h 2m
- 6. Derivatives of Inverse, Exponential, & Logarithmic Functions2h 37m
- 7. Antiderivatives & Indefinite Integrals1h 26m
- 8. Definite Integrals4h 44m
- 9. Graphical Applications of Integrals2h 27m
- 10. Physics Applications of Integrals 2h 22m
4. Applications of Derivatives
Related Rates
Problem 54
Textbook Question
Robert Boyle (1627–1691) found that for a given quantity of gas at a constant temperature, the pressure P (in kPa) and volume V of the gas (in m³) are accurately approximated by the equation V=k/P, where k>0 is constant. Suppose the volume of an expanding gas is increasing at a rate of 0.15 m³/min when the volume V=0.5 m³ and the pressure is P=50 kPa. At what rate is pressure changing at this moment?
 Verified step by step guidance
Verified step by step guidance1
Start by understanding Boyle's Law, which states that for a given quantity of gas at constant temperature, the product of pressure and volume is constant: V = k/P. Here, k is a constant.
Given that the volume V is increasing at a rate of 0.15 m³/min, we need to find the rate at which the pressure P is changing. This involves differentiating the equation V = k/P with respect to time t.
Differentiate both sides of the equation V = k/P with respect to time t. Use the chain rule for differentiation: dV/dt = -k/P² * dP/dt.
Substitute the known values into the differentiated equation. You have dV/dt = 0.15 m³/min, V = 0.5 m³, and P = 50 kPa. Also, find the constant k using the equation V = k/P, which gives k = V * P.
Solve for dP/dt, the rate of change of pressure, using the substituted values in the differentiated equation. Rearrange the equation to isolate dP/dt and calculate its value.
 Verified video answer for a similar problem:
Verified video answer for a similar problem:This video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
Was this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Related Rates
Related rates involve finding the rate at which one quantity changes in relation to another. In this problem, we need to determine how the pressure of the gas changes as its volume changes. This requires applying the chain rule of differentiation to relate the rates of change of volume and pressure.
Recommended video:
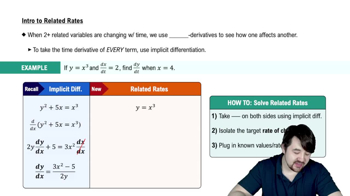
Intro To Related Rates
Implicit Differentiation
Implicit differentiation is a technique used to differentiate equations where the variables are not isolated. In this case, we will differentiate the equation V = k/P with respect to time to find the relationship between the rates of change of volume and pressure. This allows us to solve for the unknown rate of change of pressure.
Recommended video:
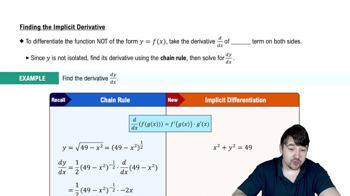
Finding The Implicit Derivative
Boyle's Law
Boyle's Law states that for a given mass of gas at constant temperature, the product of pressure and volume is a constant (PV = k). This relationship is crucial for understanding how changes in volume affect pressure. In this problem, we use the equation V = k/P to analyze the behavior of the gas as its volume increases.
Related Videos
Related Practice





