Textbook Question
When you hold your breath, which of the following blood gas changes first leads to the urge to breathe? a. rising O2 b. falling O2 c. rising CO2 c, falling CO2
790
views
 Verified step by step guidance
Verified step by step guidance
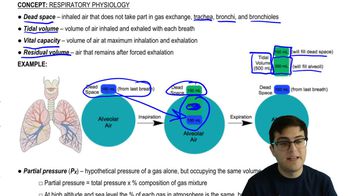

When you hold your breath, which of the following blood gas changes first leads to the urge to breathe? a. rising O2 b. falling O2 c. rising CO2 c, falling CO2
One feature that amphibians and humans have in common is a. the number of heart chambers. b. a complete separation of circuits for circulation. c. the number of circuits for circulation. d. a low blood pressure in the systemic circuit.
If a molecule of CO2 released into the blood in your left toe is exhaled from your nose, it must pass through all of the following except a. the pulmonary vein. b. the trachea. c. the right atrium. d. the right ventricle.