Properties of Water- Cohesion and Adhesion quiz Flashcards
 Back
BackTerms in this set (19)
What is cohesion in the context of water properties?
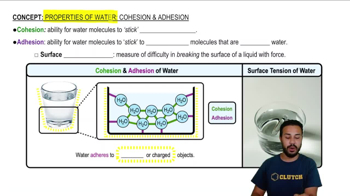
Cohesion is the ability of water molecules to stick to each other due to hydrogen bonding.
What does adhesion refer to in terms of water molecules?
Adhesion is the ability of water molecules to stick to other substances that are not water, such as glass.
How do hydrogen bonds contribute to the cohesion of water?
Hydrogen bonds form between water molecules, allowing them to stick together, which is referred to as cohesion.
What type of molecules does water adhere to?
Water adheres to polar and charged molecules.
What is surface tension, and how is it related to cohesion and adhesion?
Surface tension is a measure of the difficulty in breaking the surface of a liquid, created by the cohesive and adhesive properties of water.
Why can a paperclip float on the surface of water despite being denser than water?
A paperclip can float on water due to the surface tension created by the cohesion and adhesion of water molecules.
What role does polarity play in the cohesion and adhesion of water?
Water's polarity allows it to form hydrogen bonds, which are essential for both cohesion and adhesion.
Can water adhere to non-polar molecules?
No, water typically adheres to polar and charged molecules.
What is the relationship between surface tension and the force required to break the surface of water?
Surface tension indicates the amount of force needed to break the surface of water, which is influenced by cohesion and adhesion.
How do organisms take advantage of water's surface tension?
Some organisms can exploit water's surface tension to move or rest on the water surface without sinking.
What is the root meaning of 'co' in cohesion?
The root 'co' in cohesion means 'together,' indicating water molecules sticking together.
Why is glass considered a polar object in the context of water adhesion?
Glass is considered polar because it has charged regions that allow water molecules to adhere to it.
What happens when enough force is applied to a paperclip on the surface of water?
When enough force is applied, the paperclip breaks the surface tension and sinks.
- which of the following is most directly responsible for water’s unique properties?Water's unique properties are most directly due to its ability to form hydrogen bonds.
- which of the following best describes a result of the polar nature of water molecules?The polar nature of water molecules results in cohesion and adhesion, allowing water to stick to itself and other polar surfaces.
- which of the following is responsible for the cohesive property of water?The cohesive property of water is due to hydrogen bonds between water molecules.
- which example illustrates adhesion?An example of adhesion is water molecules sticking to a glass surface.
- water moves up a plant because of hydrogen bonds by a process called __________.Water moves up a plant by a process called capillary action, which involves cohesion and adhesion.
- which of the following biological activities best demonstrates water’s adhesive properties?Water's adhesive properties are best demonstrated by its ability to move through plant vessels, adhering to the walls of the vessels.