Back
BackpH Scale exam
Terms in this set (29)
pH Scale
A scale ranging from 0 to 14 that measures hydrogen ion concentration.
What does a pH below 7 indicate?
Acidity
What does a pH above 7 signify?
Basicity
Neutral pH
A pH of 7
Buffers
Substances that resist changes in pH when acids or bases are added.
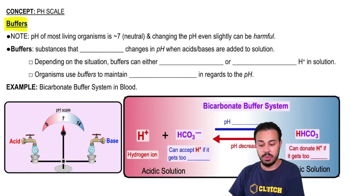
Bicarbonate Buffer System
A buffer system in blood that helps maintain pH around 7.
Why is pH important for biological processes?
Because many biological processes are strongly affected by hydrogen ion concentration.
What happens when pH is lower than 7?
The solution is acidic.
What happens when pH is higher than 7?
The solution is basic.
Inverse Proportionality in pH
When pH is low, hydrogen ion concentration is high, and vice versa.
Hydroxide Ion Concentration
Directly proportional to pH; higher pH means higher hydroxide ion concentration.
What is the pH of pure water?
7 (Neutral)
Effect of pH on living organisms
Even slight pH shifts can be harmful.
How do buffers maintain pH?
By either accepting or donating hydrogen ions.
What is the pH of lemon juice?
Around 2
What is the pH of ocean water?
About 8 (slightly basic)
What is the pH of ammonia?
About 12 (basic)
What is the pH of black coffee?
Around 5 (slightly acidic)
What does a high pH indicate about hydrogen ion concentration?
Low hydrogen ion concentration
What does a low pH indicate about hydrogen ion concentration?
High hydrogen ion concentration
Homeostasis
The maintenance of stable internal conditions, such as pH.
What is the role of HCO3-in the bicarbonate buffer system?
It can accept hydrogen ions to decrease their concentration.
What is the role of HHCO3 in the bicarbonate buffer system?
It can donate hydrogen ions to increase their concentration.
Why do living organisms need buffers?
To maintain pH around 7 and prevent harmful pH changes.
What is the pH of battery acid?
Very low (highly acidic)
What is the pH of baking soda?
Slightly basic
What is the pH of milk?
Slightly acidic
What is the pH of drain cleaner?
About 14 (highly basic)
What does the pH scale measure?
Hydrogen ion concentration in a solution.