2. Chemistry
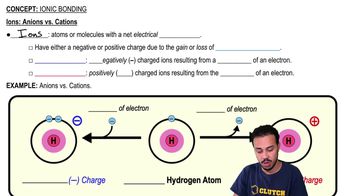
Ionic Bonding
2. Chemistry
Ionic Bonding
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
When atoms gain or lose electrons, they become negatively or positively charged. They are known as:
5752views61rank - Multiple Choice
Which of the following statements is true of ALL atoms that are anions?
6427views71rank - Multiple Choice
If oxygen has 9 electrons it will be a ______________________:
4757views78rank - Multiple Choice
An ionic bond is a bond in which:
4862views67rank