In this video, we're going to introduce the Michaelis constant or the Michaelis constant of an enzyme. The Michaelis constant is an enzyme kinetics variable that's abbreviated as just KM. The KM or the Michaelis constant, as we'll see moving forward in our course, can be defined in several different ways. As we move forward in our course, we'll talk about all of those different ways to define the Michaelis constant or the Michaelis constant. But as we can see in this lesson video, one of the ways that we can define the Michaelis constant or the Michaelis constant is as an exact substrate concentration. In fact, the Michaelis constant is the exact substrate concentration at which the initial reaction velocity, or the V0, is exactly equal to one half of the theoretical maximal reaction velocity or the Vmax. The Michaelis constant is an exact substrate concentration, so we can say that when the value of the substrate concentration active sites are going to be full or occupied with substrate to form the enzyme-substrate complex. Let’s take a look at our image down below to clear some of this up. Notice on the right, what we have is our enzyme kinetics plot that we've seen so many times in our previous lesson videos. Notice on the y-axis, what we have is the initial reaction velocity, and on the x-axis, what we have is the substrate concentration in units of molarity. We can see that our green curve right here is the same curve that we've seen so many times before in our previous lesson videos. We already know from our previous lesson videos on the V_max of an enzyme that the Vmax acts as a horizontal asymptote to limit the initial reaction velocity of an enzyme-catalyzed reaction. We can see that this curve will get very close to the Vmax, but it's not actually attained by any enzyme. That's why we said that the Vmax is better defined as a theoretical maximal reaction velocity. The Vmax can only be attained under saturating substrate concentrations where the substrate concentration is very high and is the only way where the initial reaction velocity can approach the Vmax. So the question is, how do we determine the substrate concentration that's needed to get half of all of the available enzyme active sites occupied? Well, it's pretty easy. All we need to do is find the value of the Vmax and then cut that value in half, or essentially take the Vmax over 2. The point on our curve that corresponds with half of the Vmax correlates with the substrate concentration that equals the value of the Michaelis constant. The Michaelis constant is really just expressed as an exact substrate concentration, and it's the exact substrate concentration that's needed to get the initial reaction velocity equal to exactly half of the Vmax. It's important to note here is that the Michaelis constant is only associated with half of the Vmax, but the Michaelis constant is not equal to the value of half of the Vmax. The Michaelis constant is an exact substrate concentration, so it has units of substrate concentration, or units of concentration. Another way to look at this Michaelis constant here is, as we mentioned already up above in our lesson, when the value of the substrate concentration is equal to the value of the K_M, we can say that exactly half or fifty percent of all of the available enzyme active sites are going to be full or occupied with substrate. Notice over here what we have are enzymes as these brown structures, and these little dots here are our substrate, and notice that we have a total of 6 enzymes here. When the value of the substrate concentration is equal to the value of the Michaelis constant, notice that exactly half or fifty percent of all of these enzymes here are actually occupied or full with substrate to form the enzyme-substrate complex. What we can say again is that when the substrate concentration value is equal to the value of the Michaelis constant, that exactly half of all of the available enzymes or half of the total concentration of enzyme is going to equal the concentration of the enzyme-substrate complex. It's also important to note, as we move forward in our course, we'll talk more about this idea later, but it's important to note now that the Michaelis constant is an intrinsic property of an enzyme, which means that it does not get affected by the amount of enzyme that's present. It's a property of the enzyme that won't change, regardless of how much enzyme is present. The K_M of an enzyme can change depending on the conditions such as different pHs, different temperatures, or different solvents. This concludes our introduction to the Michaelis constant, and again, as we continue to move forward in our course, we'll talk more and more about this Michaelis constant. I'll see you guys in our next video.
- 1. Introduction to Biochemistry4h 34m
- What is Biochemistry?5m
- Characteristics of Life12m
- Abiogenesis13m
- Nucleic Acids16m
- Proteins12m
- Carbohydrates8m
- Lipids10m
- Taxonomy10m
- Cell Organelles12m
- Endosymbiotic Theory11m
- Central Dogma22m
- Functional Groups15m
- Chemical Bonds13m
- Organic Chemistry31m
- Entropy17m
- Second Law of Thermodynamics11m
- Equilibrium Constant10m
- Gibbs Free Energy37m
- 2. Water3h 23m
- 3. Amino Acids8h 10m
- Amino Acid Groups8m
- Amino Acid Three Letter Code13m
- Amino Acid One Letter Code37m
- Amino Acid Configuration20m
- Essential Amino Acids14m
- Nonpolar Amino Acids21m
- Aromatic Amino Acids14m
- Polar Amino Acids16m
- Charged Amino Acids40m
- How to Memorize Amino Acids1h 7m
- Zwitterion33m
- Non-Ionizable Vs. Ionizable R-Groups11m
- Isoelectric Point10m
- Isoelectric Point of Amino Acids with Ionizable R-Groups51m
- Titrations of Amino Acids with Non-Ionizable R-Groups44m
- Titrations of Amino Acids with Ionizable R-Groups38m
- Amino Acids and Henderson-Hasselbalch44m
- 4. Protein Structure10h 4m
- Peptide Bond18m
- Primary Structure of Protein31m
- Altering Primary Protein Structure15m
- Drawing a Peptide44m
- Determining Net Charge of a Peptide42m
- Isoelectric Point of a Peptide37m
- Approximating Protein Mass7m
- Peptide Group22m
- Ramachandran Plot26m
- Atypical Ramachandran Plots12m
- Alpha Helix15m
- Alpha Helix Pitch and Rise20m
- Alpha Helix Hydrogen Bonding24m
- Alpha Helix Disruption23m
- Beta Strand12m
- Beta Sheet12m
- Antiparallel and Parallel Beta Sheets39m
- Beta Turns26m
- Tertiary Structure of Protein16m
- Protein Motifs and Domains23m
- Denaturation14m
- Anfinsen Experiment20m
- Protein Folding34m
- Chaperone Proteins19m
- Prions4m
- Quaternary Structure15m
- Simple Vs. Conjugated Proteins10m
- Fibrous and Globular Proteins11m
- 5. Protein Techniques14h 5m
- Protein Purification7m
- Protein Extraction5m
- Differential Centrifugation15m
- Salting Out18m
- Dialysis9m
- Column Chromatography11m
- Ion-Exchange Chromatography35m
- Anion-Exchange Chromatography38m
- Size Exclusion Chromatography28m
- Affinity Chromatography16m
- Specific Activity16m
- HPLC29m
- Spectrophotometry51m
- Native Gel Electrophoresis23m
- SDS-PAGE34m
- SDS-PAGE Strategies16m
- Isoelectric Focusing17m
- 2D-Electrophoresis23m
- Diagonal Electrophoresis29m
- Mass Spectrometry12m
- Mass Spectrum47m
- Tandem Mass Spectrometry16m
- Peptide Mass Fingerprinting16m
- Overview of Direct Protein Sequencing30m
- Amino Acid Hydrolysis10m
- FDNB26m
- Chemical Cleavage of Bonds29m
- Peptidases1h 6m
- Edman Degradation30m
- Edman Degradation Sequenator and Sequencing Data Analysis4m
- Edman Degradation Reaction Efficiency20m
- Ordering Cleaved Fragments21m
- Strategy for Ordering Cleaved Fragments58m
- Indirect Protein Sequencing Via Geneomic Analyses24m
- 6. Enzymes and Enzyme Kinetics13h 38m
- Enzymes24m
- Enzyme-Substrate Complex17m
- Lock and Key Vs. Induced Fit Models23m
- Optimal Enzyme Conditions9m
- Activation Energy24m
- Types of Enzymes41m
- Cofactor15m
- Catalysis19m
- Electrostatic and Metal Ion Catalysis11m
- Covalent Catalysis18m
- Reaction Rate10m
- Enzyme Kinetics24m
- Rate Constants and Rate Law35m
- Reaction Orders52m
- Rate Constant Units11m
- Initial Velocity31m
- Vmax Enzyme27m
- Km Enzyme42m
- Steady-State Conditions25m
- Michaelis-Menten Assumptions18m
- Michaelis-Menten Equation52m
- Lineweaver-Burk Plot43m
- Michaelis-Menten vs. Lineweaver-Burk Plots20m
- Shifting Lineweaver-Burk Plots37m
- Calculating Vmax40m
- Calculating Km31m
- Kcat46m
- Specificity Constant1h 1m
- 7. Enzyme Inhibition and Regulation 8h 42m
- Enzyme Inhibition13m
- Irreversible Inhibition12m
- Reversible Inhibition9m
- Inhibition Constant26m
- Degree of Inhibition15m
- Apparent Km and Vmax29m
- Inhibition Effects on Reaction Rate10m
- Competitive Inhibition52m
- Uncompetitive Inhibition33m
- Mixed Inhibition40m
- Noncompetitive Inhibition26m
- Recap of Reversible Inhibition37m
- Allosteric Regulation7m
- Allosteric Kinetics17m
- Allosteric Enzyme Conformations33m
- Allosteric Effectors18m
- Concerted (MWC) Model25m
- Sequential (KNF) Model20m
- Negative Feedback13m
- Positive Feedback15m
- Post Translational Modification14m
- Ubiquitination19m
- Phosphorylation16m
- Zymogens13m
- 8. Protein Function 9h 41m
- Introduction to Protein-Ligand Interactions15m
- Protein-Ligand Equilibrium Constants22m
- Protein-Ligand Fractional Saturation32m
- Myoglobin vs. Hemoglobin27m
- Heme Prosthetic Group31m
- Hemoglobin Cooperativity23m
- Hill Equation21m
- Hill Plot42m
- Hemoglobin Binding in Tissues & Lungs31m
- Hemoglobin Carbonation & Protonation19m
- Bohr Effect23m
- BPG Regulation of Hemoglobin24m
- Fetal Hemoglobin6m
- Sickle Cell Anemia24m
- Chymotrypsin18m
- Chymotrypsin's Catalytic Mechanism38m
- Glycogen Phosphorylase21m
- Liver vs Muscle Glycogen Phosphorylase21m
- Antibody35m
- ELISA15m
- Motor Proteins14m
- Skeletal Muscle Anatomy22m
- Skeletal Muscle Contraction45m
- 9. Carbohydrates7h 49m
- Carbohydrates19m
- Monosaccharides15m
- Stereochemistry of Monosaccharides33m
- Monosaccharide Configurations32m
- Cyclic Monosaccharides20m
- Hemiacetal vs. Hemiketal19m
- Anomer14m
- Mutarotation13m
- Pyranose Conformations23m
- Common Monosaccharides33m
- Derivatives of Monosaccharides21m
- Reducing Sugars21m
- Reducing Sugars Tests19m
- Glycosidic Bond48m
- Disaccharides40m
- Glycoconjugates12m
- Polysaccharide7m
- Cellulose7m
- Chitin8m
- Peptidoglycan12m
- Starch13m
- Glycogen14m
- Lectins16m
- 10. Lipids5h 49m
- Lipids15m
- Fatty Acids30m
- Fatty Acid Nomenclature11m
- Omega-3 Fatty Acids12m
- Triacylglycerols11m
- Glycerophospholipids24m
- Sphingolipids13m
- Sphingophospholipids8m
- Sphingoglycolipids12m
- Sphingolipid Recap22m
- Waxes5m
- Eicosanoids19m
- Isoprenoids9m
- Steroids14m
- Steroid Hormones11m
- Lipid Vitamins19m
- Comprehensive Final Lipid Map13m
- Biological Membranes16m
- Physical Properties of Biological Membranes18m
- Types of Membrane Proteins8m
- Integral Membrane Proteins16m
- Peripheral Membrane Proteins12m
- Lipid-Linked Membrane Proteins21m
- 11. Biological Membranes and Transport 6h 37m
- Biological Membrane Transport21m
- Passive vs. Active Transport18m
- Passive Membrane Transport21m
- Facilitated Diffusion8m
- Erythrocyte Facilitated Transporter Models30m
- Membrane Transport of Ions29m
- Primary Active Membrane Transport15m
- Sodium-Potassium Ion Pump20m
- SERCA: Calcium Ion Pump10m
- ABC Transporters12m
- Secondary Active Membrane Transport12m
- Glucose Active Symporter Model19m
- Endocytosis & Exocytosis18m
- Neurotransmitter Release23m
- Summary of Membrane Transport21m
- Thermodynamics of Membrane Diffusion: Uncharged Molecule51m
- Thermodynamics of Membrane Diffusion: Charged Ion1h 1m
- 12. Biosignaling9h 45m
- Introduction to Biosignaling44m
- G protein-Coupled Receptors32m
- Stimulatory Adenylate Cyclase GPCR Signaling42m
- cAMP & PKA28m
- Inhibitory Adenylate Cyclase GPCR Signaling29m
- Drugs & Toxins Affecting GPCR Signaling20m
- Recap of Adenylate Cyclase GPCR Signaling5m
- Phosphoinositide GPCR Signaling58m
- PSP Secondary Messengers & PKC27m
- Recap of Phosphoinositide Signaling7m
- Receptor Tyrosine Kinases26m
- Insulin28m
- Insulin Receptor23m
- Insulin Signaling on Glucose Metabolism57m
- Recap Of Insulin Signaling in Glucose Metabolism6m
- Insulin Signaling as a Growth Factor1h 1m
- Recap of Insulin Signaling As A Growth Factor9m
- Recap of Insulin Signaling1m
- Jak-Stat Signaling25m
- Lipid Hormone Signaling15m
- Summary of Biosignaling13m
- Signaling Defects & Cancer20m
- Review 1: Nucleic Acids, Lipids, & Membranes2h 47m
- Nucleic Acids 19m
- Nucleic Acids 211m
- Nucleic Acids 34m
- Nucleic Acids 44m
- DNA Sequencing 19m
- DNA Sequencing 211m
- Lipids 111m
- Lipids 24m
- Membrane Structure 110m
- Membrane Structure 29m
- Membrane Transport 18m
- Membrane Transport 24m
- Membrane Transport 36m
- Practice - Nucleic Acids 111m
- Practice - Nucleic Acids 23m
- Practice - Nucleic Acids 39m
- Lipids11m
- Practice - Membrane Structure 17m
- Practice - Membrane Structure 25m
- Practice - Membrane Transport 16m
- Practice - Membrane Transport 26m
- Review 2: Biosignaling, Glycolysis, Gluconeogenesis, & PP-Pathway3h 12m
- Biosignaling 19m
- Biosignaling 219m
- Biosignaling 311m
- Biosignaling 49m
- Glycolysis 17m
- Glycolysis 27m
- Glycolysis 38m
- Glycolysis 410m
- Fermentation6m
- Gluconeogenesis 18m
- Gluconeogenesis 27m
- Pentose Phosphate Pathway15m
- Practice - Biosignaling13m
- Practice - Bioenergetics 110m
- Practice - Bioenergetics 216m
- Practice - Glycolysis 111m
- Practice - Glycolysis 27m
- Practice - Gluconeogenesis5m
- Practice - Pentose Phosphate Path6m
- Review 3: Pyruvate & Fatty Acid Oxidation, Citric Acid Cycle, & Glycogen Metabolism2h 26m
- Pyruvate Oxidation9m
- Citric Acid Cycle 114m
- Citric Acid Cycle 27m
- Citric Acid Cycle 37m
- Citric Acid Cycle 411m
- Metabolic Regulation 18m
- Metabolic Regulation 213m
- Glycogen Metabolism 16m
- Glycogen Metabolism 28m
- Fatty Acid Oxidation 111m
- Fatty Acid Oxidation 28m
- Citric Acid Cycle Practice 17m
- Citric Acid Cycle Practice 26m
- Citric Acid Cycle Practice 32m
- Glucose and Glycogen Regulation Practice 14m
- Glucose and Glycogen Regulation Practice 26m
- Fatty Acid Oxidation Practice 14m
- Fatty Acid Oxidation Practice 27m
- Review 4: Amino Acid Oxidation, Oxidative Phosphorylation, & Photophosphorylation1h 48m
- Amino Acid Oxidation 15m
- Amino Acid Oxidation 211m
- Oxidative Phosphorylation 18m
- Oxidative Phosphorylation 210m
- Oxidative Phosphorylation 310m
- Oxidative Phosphorylation 47m
- Photophosphorylation 15m
- Photophosphorylation 29m
- Photophosphorylation 310m
- Practice: Amino Acid Oxidation 12m
- Practice: Amino Acid Oxidation 22m
- Practice: Oxidative Phosphorylation 15m
- Practice: Oxidative Phosphorylation 24m
- Practice: Oxidative Phosphorylation 35m
- Practice: Photophosphorylation 15m
- Practice: Photophosphorylation 21m
Km Enzyme - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AIThe Michaelis constant (Km) is a crucial parameter in enzyme kinetics, representing the substrate concentration at which the initial reaction velocity (V0) is half of the maximum velocity (Vmax). It can be expressed as a ratio of rate constants under steady-state conditions, indicating an enzyme's binding affinity for its substrate. A higher Km signifies weaker affinity, while a lower Km indicates stronger affinity. Importantly, Km remains constant regardless of enzyme concentration, highlighting its intrinsic nature.
Km Enzyme
Video transcript
What is the initial velocity of a reaction when the concentration of substrate is set equal to the Km?
Km Enzyme
Video transcript
So we already know at this point that the Michaelis constant, or the kilometers, can actually be expressed in multiple ways. And in our last lesson video, we literally said that the kilometers was a substrate concentration. More specifically, what we said was that the kilometers was the exact substrate concentration that allows for the initial reaction velocity. The Michaelis constant, \( k_m \), can also be defined as a substrate concentration when it is expressed with rate constants. What I want you guys to recall from our previous lesson videos is that reaction rates or reaction velocities, symbolized with \( v \), can be expressed by rate laws. We know that rate laws incorporate rate constants, which are abbreviated with the lowercase letter \( k \). And, of course, we also already know from our previous lesson videos that initially, at the very, very, very beginning of an enzyme catalyzed reaction, there are only 3 relevant rate constants.
Down below in our image here of our enzyme catalyzed reaction, we can see that this is the reaction initially at the very beginning of the enzyme catalyzed reaction, and we know that because there are only 3 relevant rate constants, \( k_1 \), \( k_{-1} \), and \( k_2 \), and the reverse rate constant here, for \( k_{-2} \), is negligible at the very beginning of the enzyme catalyzed reaction. That's why we have this initial reaction velocity symbol here, to help remind you guys that this is at the very beginning. Now, all of this is pretty much review from our previous lesson video. So what I want you guys to know in this video is that the Michaelis constant, kilometers, can also be defined as just a compilation of these 3 rate constants that we're already familiar with from our previous lesson videos.
Now, I'll admit at first glance, this ratio might look pretty random, but this ratio is actually not random at all. In fact, this ratio is defined specifically under conditions known as steady state conditions, which we'll talk more about later in our course. But for now, all I want you guys to know is that this ratio right here that defines the Michaelis constant, kilometers, is not random, and it's actually defined under steady state conditions. As random as this ratio might appear, if we take an even closer look at this ratio, we'll see that it's just the ratio of the sum of the 2 dissociation rate constants for the enzyme substrate complex over the association rate constant for the enzyme substrate complex.
That's exactly what we're saying down below: that the Michaelis kasukm is defined as the enzyme substrate complex dissociation over the enzyme substrate complex association. The enzyme substrate complex can actually dissociate in 2 different ways. It can dissociate backwards via \( k_{-1} \) to form the free enzyme and free substrate, or the enzyme substrate complex could also dissociate forward via \( k_2 \) to form the free enzyme and the free product. That's why we can take \( k_{-1} \) and \( k_2 \) and sum them together to get our rate, to get our dissociation of the enzyme substrate complex. Then, of course, we know that the enzyme substrate complex can only associate in this reaction here via \( k_1 \), and that's why for the association at the bottom, we place in \( k_1 \).
From our last lesson video, we said that the kilometers was an exact substrate concentration. Even when the kilometers is expressed as this ratio of rate constants, it still represents that same substrate concentration. I can prove it to you guys just by looking at the units. The Michaelis constant, or the Kilometers, even when it's expressed as this ratio of rate constants, still has units that are equal to molarity, and molarity is, of course, a unit of concentration, which shows that the kilometers is still a substrate concentration. We could have actually determined this by using previous knowledge acquired from our previous lesson video.
We know that both \( k_{-1} \) and \( k_2 \) only have one reactant, which is the enzyme substrate complex. Because they only have one reactant, they are both first order rate constants. We know from our previous lesson videos that first order rate constants have units of inverse seconds. So, focusing on the units: \( \text{inverse seconds} + \text{inverse seconds} \), the final units on the top number, if focusing only on the units, are still just going to be inverse seconds. And then, of course, the bottom still gonna have inverse molarity, inverse seconds. What you end up with is that the units of inverse seconds cancel out, and then what you get is \( 1 / \text{inverse molarity} \), which is the same exact thing as saying molarity.
This compilation of rate constants in this ratio here still has units of molarity, which means that Kilometers is still a substrate concentration, just like what we said in our last lesson video. It has to be the dissociation over the association of the enzyme substrate complex, and it can't be the reverse because if it were, then the units would turn out to be inverse molarity, which would mean that Kilometers has units of inverse molarity, and inverse molarity is not a unit of substrate concentration.
Later in our course, we'll be able to talk more about exactly how the kilometers is not just the substrate concentration, but an exact substrate concentration that allows for the initial reaction velocity to equal half of the \( v_{max} \). We'll talk more about that later in our course. But for now, all I want you guys to really know from this video is that the kilometers can also be expressed with rate constants in this format right here. So that concludes this lesson video, and I'll see you guys in our next video.
Select the best description of the K m.
Km Enzyme
Video transcript
So from our last lesson video, we know that the Michaelis constant or the \( k_m \) is just a substrate concentration, and it can be expressed with rate constants. Now, in this lesson video, one of the things that we're going to do is define yet another way to express the Michaelis constant \( k_m \). And so the \( k_m \) can also be defined or expressed as the ratio of the free enzyme concentration times the free substrate concentration over the enzyme-substrate complex concentration. And so notice down below, we can refresh our memories that the Michaelis constant \( k_m \) is just expressed as the enzyme-substrate complex dissociation over the enzyme-substrate complex association, and we know that this ratio of the rate constants defines the \( k_m \) from our last lesson video. And so here what we're saying is that the dissociated form of the enzyme-substrate complex is really just going to be the free enzyme and the free substrate. And, of course, the associated form of the enzyme-substrate complex on the bottom is just going to be the enzyme-substrate complex. And so it's this ratio here that also defines the Michaelis constant \( k_m \). And again, we already know from our previous lesson videos that even though these ratios here might seem random, they're actually not random at all and they're derived under conditions known as steady state conditions, which again, we're going to talk about a little bit later in our course. But for now, all I want you guys to know is that the Michaelis constant \( k_m \) is defined by these ratios. Now, because the \( k_m \) is defined by these ratios, it means that the \( k_m \) measures an enzyme's binding affinity for its substrate. By affinity, what we mean is the strength of its attraction. Affinity is essentially the same as the attraction. And so it turns out that the Michaelis constant \( k_m \) actually has an inverse relationship with an enzyme's binding affinity for its substrate. And so what I mean by that is that the larger the value of the \( K_m \) is, the smaller the binding affinity an enzyme has for its substrate, or the weaker the binding affinity an enzyme has for its substrate. And so just to clear that up, let's take a look at our image on the bottom left, down below. And so notice that when we have a large value of the \( K_m \), indicated by this large upward arrow and the large size of the \( K_m \), notice that that means that the dissociation of the enzyme-substrate complex is going to be quite large, and, of course, this means that the association of the enzyme-substrate complex is going to be quite small. And so if the enzyme-substrate complex is dissociating a lot, that means that it's breaking apart a lot, and that must mean that the enzyme does not have a very strong affinity for its substrate. And so this means that when we have a large \( K_m \), that the enzyme's affinity for its substrate will be weak, and so we will have a weak affinity. Now with the reverse scenario, if we have a small \( K_m \), the value of the \( K_m \) indicated by the small downward arrow and the small size of the \( K_m \). Notice this means that this time the dissociation of the enzyme-substrate complex is going to be quite small, and instead the association of the enzyme-substrate complex is going to be quite large. And so of course, if there's a lot of association of the enzyme-substrate complex going on, that means that the enzyme and the substrate will be associating together to form the enzyme-substrate complex, and that means that the enzyme must have a strong affinity for the substrate. And so that is exactly what a small \( K_m \) indicates, is a strong affinity that the enzyme has for its substrate. And so notice that in this example problem here, it's asking us which enzyme has a stronger affinity for its substrate. Is it enzyme a or is it enzyme b? And down below, we're given this enzyme kinetics plot here, where we have the initial reaction rate, or \( V_0 \) on the y-axis, and the substrate concentration this, the magnitude of the \( K_m \) that dictates the strength of the affinity. And so what going to want to do is determine the \( K_m \) for both of these enzymes. So notice that we have two curves here. We have this red curve here for enzyme b, and then we have this blue curve here for enzyme a. So we need to determine the \( K_m \)'s in order to determine which one has a stronger affinity for the substrate. And so if we start with enzyme B, notice that enzyme B has a \( V_{max} \) that is showing up somewhere horizontally over here. And so, we know that the \( K_m \) from our previous lesson videos is a substrate concentration. In fact, it's the substrate concentration that allows the initial reaction velocity to equal half of the \( V_{max} \). And so, if here we have our \( V_{max} \), half of the \( V_{max} \) is going to be somewhere over here. And so that means that the point here on our curve that corresponds with half of the \( V_{max} \) is going to be really really low and associate, somewhere over here. So the red and enzyme B has a \( K_m \) that is really, really low and corresponds somewhere over here. Now, if we take a look at enzyme A, notice that its \( V_{max} \) is somewhere way up here, and so half of the \( V_{max} \) is going to be somewhere about, that corresponds somewhere around here, and that corresponds with this point, and this point here corresponds with the \( K_m \) that's right around this region right here. And so by doing this, we can clearly see that the \( K_m \) of enzyme A is actually greater than, it's larger than the \( K_m \) of enzyme B. And so remember that a larger \( K_m \) means a weaker affinity. So this means that enzyme a, because it has a larger value for the \( K_m \), it has a weaker affinity. And that means that, of course, \( K_m \) of enzyme B is smaller and has a stronger affinity. So that means that clearly, the enzyme that has the stronger affinity for its substrate is going to be enzyme B. And so we can go ahead and indicate that it's enzyme B here that is the correct answer for this problem. And so this concludes our lesson on how the \( K_m \), the Michaelis constant \( K_m \), can be used as a measure of an enzyme's affinity for its substrate, and we'll be able to practice utilizing these concepts here in our next couple of practice videos. So I'll see you guys there.
According to the chart below, which one of the following enzymes has the strongest affinity for its substrate?
Km Enzyme
Video transcript
So in one of our previous lesson videos, we mentioned the fact that the Michaelis Constant, or the Km, is an intrinsic property of an enzyme. And so all this means is that the Km is completely independent of the enzyme concentration. And so what this means is we can say that when the value of the substrate concentration is equal to the value of the Km, exactly half of all of the available enzyme active sites are going to be occupied with substrate, and this will be true regardless of the total enzyme concentration. And really, that's the main takeaway of this video: the fact that the total enzyme concentration does not affect the Michaelis constant Km.
And so, recall from our previous lesson videos on the Vmax of an enzyme that the total enzyme concentration does actually affect both the initial reaction velocity and the maximal reaction velocity. But what we're learning in this video, however, is that altering the total enzyme concentration does not affect the Km of an enzyme. And so if we take a look at our image down below, notice we have this enzyme kinetics plot where we have the initial reaction velocity on the y-axis and the substrate concentration on the x-axis. And notice that we have these 2 different curves in our plot. We've got this red curve right here and we've got this green curve right here. And notice the only difference between these two curves in our plot is the total amount of enzymes. So notice that the green curve has double the total amount of enzyme in comparison to the red curve below. And so, again, as we already know from our previous lesson videos on the Vmax of an enzyme, doubling or changing the total enzyme concentration is also going to alter both the initial reaction velocity shown by the change in the curve, and it's going to change the maximal reaction velocity in comparison to this one that's down below.
And so what we're learning again in this video is that even though varying the total enzyme concentration changes both the initial and the maximal reaction velocity, it does not change the Km. And so if we take a look at the Km here, notice that it has the same exact value for both of these curves, the red and the green curve, despite the fact that they have different amounts of total enzyme concentration. And so, notice that the Km is exactly the same. However, notice that half the Vmax is going to be different for both of these curves, and that's because the Vmax itself is different for both of these curves. However, because the Km stays the same, that tells us that the Km is an intrinsic property of the enzyme that's independent of the total enzyme concentration.
So that concludes our lesson and we'll be able to apply these concepts as we move forward in our course. So I'll see you guys in our next video.
Indicate which region of the Enzyme Kinetics plot below best corresponds to each statement.

A) Initial reaction velocity is limited mainly by the [S] present: ______
B) Initial reaction velocity limited mainly by the [E] present: ______
C) The active site of an enzyme is most likely free/unoccupied: ______
D) The active site of an enzyme is most likely occupied by substrate: ______
E) This region includes the points corresponding to Km & ½Vmax: ______
Problem Transcript
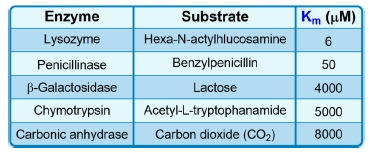
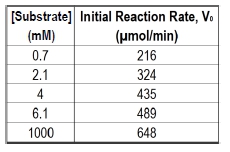
Use the data in the following chart to determine the Km of the enzyme.
Here’s what students ask on this topic:
What is the Michaelis constant (Km) in enzyme kinetics?
The Michaelis constant (Km) is a key parameter in enzyme kinetics that represents the substrate concentration at which the initial reaction velocity (V0) is half of the maximum velocity (Vmax). It provides insight into the enzyme's affinity for its substrate; a lower Km indicates higher affinity, while a higher Km suggests lower affinity. Km is intrinsic to the enzyme and remains constant regardless of enzyme concentration, although it can vary with different environmental conditions such as pH and temperature.
 Created using AI
Created using AIHow is Km related to enzyme affinity for its substrate?
Km is inversely related to an enzyme's affinity for its substrate. A lower Km value indicates a higher affinity, meaning the enzyme binds the substrate more tightly and efficiently. Conversely, a higher Km value suggests a lower affinity, indicating weaker binding between the enzyme and substrate. This relationship helps in understanding how effectively an enzyme can catalyze a reaction at varying substrate concentrations.
 Created using AI
Created using AIHow can Km be expressed using rate constants?
Km can be expressed as a ratio of rate constants under steady-state conditions. The formula is:
Here, k1 is the rate constant for the formation of the enzyme-substrate complex, k-1 is the rate constant for its dissociation back to enzyme and substrate, and k2 is the rate constant for the formation of product from the enzyme-substrate complex. This ratio reflects the balance between the formation and breakdown of the enzyme-substrate complex.
 Created using AI
Created using AIDoes Km change with enzyme concentration?
No, Km is an intrinsic property of the enzyme and does not change with enzyme concentration. It remains constant regardless of how much enzyme is present. However, Km can vary under different environmental conditions such as changes in pH, temperature, or solvent. This constancy makes Km a reliable measure of an enzyme's affinity for its substrate under specific conditions.
 Created using AI
Created using AIWhat is the significance of Km in enzyme kinetics?
Km is significant in enzyme kinetics because it provides valuable information about the enzyme's efficiency and affinity for its substrate. It helps in determining the substrate concentration required to achieve half of the maximum reaction velocity (Vmax). This information is crucial for understanding enzyme behavior in different conditions and for designing experiments and drugs that target specific enzymes. Additionally, Km can be used to compare the catalytic efficiency of different enzymes or the same enzyme under varying conditions.
 Created using AI
Created using AI