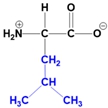
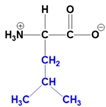
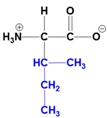
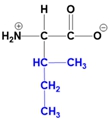
Understanding the structure of amino acids is fundamental in biochemistry, particularly the concept of zwitterions. At physiological pH, which is approximately 7, all 20 amino acids predominantly exist as zwitterions. A zwitterion is defined as a dipolar molecule that contains two groups with opposite charges, resulting from acid-base reactions or proton transfers.
In the common structure of amino acids, the backbone consists of an alpha carbon, a central hydrogen atom, a carboxylate group, an amino group, and a variable R group. At physiological pH, the amino group carries a positive charge, while the carboxylate group has a negative charge due to the loss of a proton. This dual charge character is what classifies the amino acid as a zwitterion.
The significance of zwitterions extends beyond mere classification; they play a crucial role in the behavior of amino acids in biological systems, influencing solubility, reactivity, and interactions with other biomolecules. Recognizing that the zwitterionic form is the predominant structure at physiological pH is essential for understanding protein structure and function, as well as the overall chemistry of amino acids.
As you continue your studies, keep in mind that this foundational knowledge about zwitterions will be referenced frequently, enhancing your comprehension of more complex biochemical concepts.