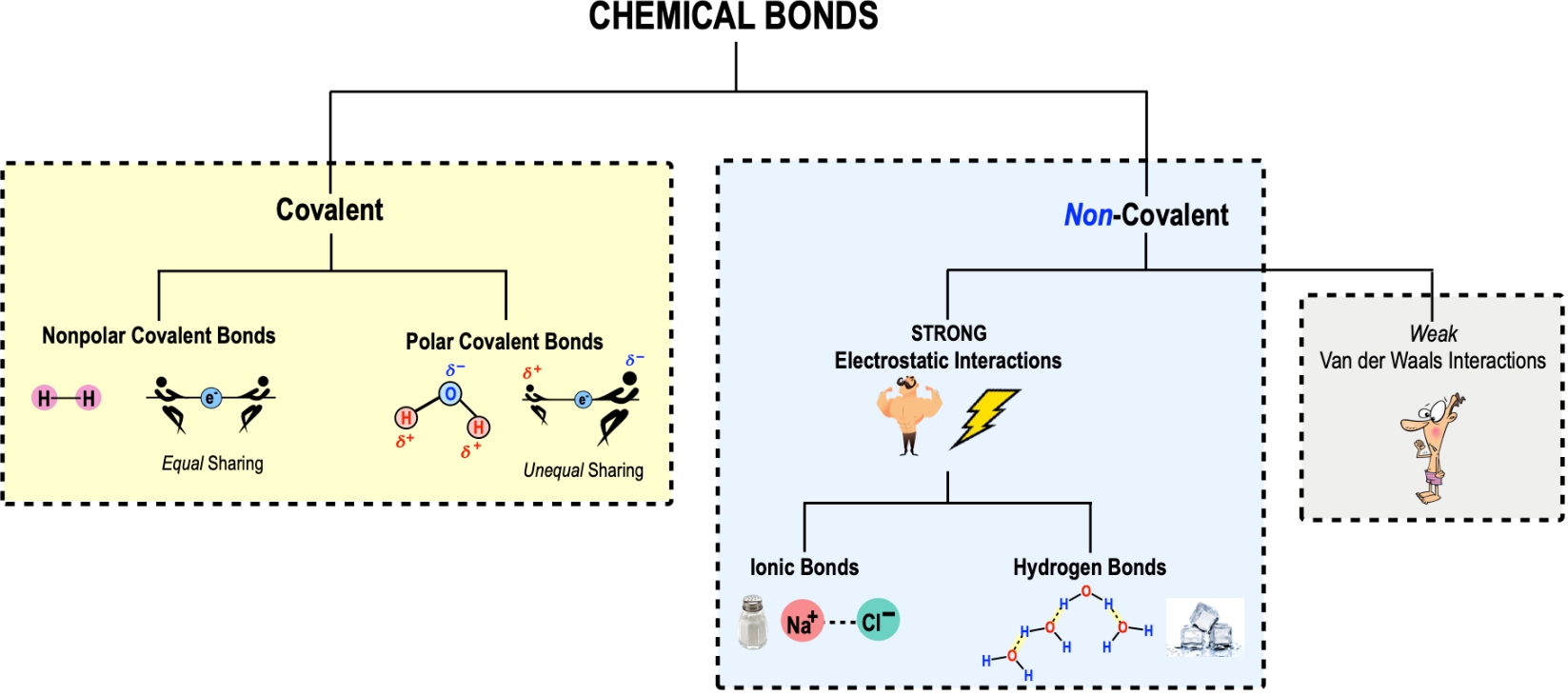
In this video, we're going to begin our introduction to chemical bonding. And so chemical bonds are really just defined as attractive forces between atoms that hold those atoms together to form either molecules and or compounds. And so molecules is really a term that is very very broad and refers to any substance that contains greater than or equal to 2 chemically bound atoms. Now these atoms could be of different elements, or these atoms could be of the same element. And so, for example, O2 is an example of a molecule which is oxygen gas.
So if we take a look at our image down below over here on the left hand side notice that we're showing you oxygen gas which can be represented like this, or it could be represented in this format right here as well. But really oxygen gas is when you have 2 oxygen atoms that are chemically bound to each other, And so because we have 2, at least 2, atoms that are bound to each other, this makes oxygen gas an example of a molecule. But there are really all different types of molecules and, you can see here in this image we're showing you how oxygen gas can be taken from the atmosphere and breathe into our lungs and we'll talk more about that process a little bit later in our course. But now to distinguish these compounds from molecules. Now compounds as their name implies with the comp part here are a little bit more complicated and that's because they are going to have at least 2 different elements.
So notice here that we're comparing the word atoms to this word elements. Molecules have at least 2 chemically bound atoms. It doesn't matter if those atoms are the same element or if they're of different elements. Either way if it has at least 2 chemically bound atoms then it will be a molecule. But compounds on the other hand as their name implies they're a little bit more complicated because not all molecules are compounds, only very specific molecules are compounds.
And so you can see that compounds are defined as complicated molecules themselves, So they are molecules, just a very specific type of molecule that is composed of at least 2 different elements. And so for example, water is going to be an example of a compound and that is because it has 2 different elements. It has the element hydrogen and it has the element oxygen. Whereas O2 here does not have 2 different elements. It only has 2 different atoms of the same element.
And so, oxygen gas is not a compound even though it is a molecule. But water here is both a molecule and a compound because it has it meets both of these requirements here. And so if we take a look at our image down below you can see that we're saying that, water is an example of a compound, once again because it has 2 different elements, hydrogen element and an oxygen element. But another example of a compound is going to be this, structure right here which is a glucose molecule. Now you'll need to know about glucose later in our course, but it's good to start familiarizing yourself with this, compound here.
Now this is a compound because notice that it has at least 2 different elements. Notice that it has the element oxygen throughout, in these locations. It has the element carbon, in these locations right here, and then it also has the element hydrogen as well. And so, this compound, has at least 2 different elements and that's what makes it a compound. And glucose is really a sugar that is going to be found in many different forms of life and in many different, mixtures too such as in honey.
Now, the last idea here that you all should know is this idea of the chemical formula. The chemical formula is really what reveals both the number and the type of atoms that are in a molecule or compound. And so an example of a chemical formula is this chemical formula that you see right here, which is the chemical formula for glucose. And so you can see that if scientists had to write out the entire structure of glucose every single time it came out, it might take a long time to do that. And so chemical formulas can help to write down structures much much faster.
So writing down C6H12O6 is much faster than drawing out this entire structure, so chemical formulas can be very very useful in that respect. So notice that here, we're taking this compound glucose and we're changing it into a chemical formula of glucose. And so, you can see that there are a total of, this says 6 carbon atoms, so we can say this part right here means that there are a total of 6 carbon atoms. This part right here with the h twelve means that there are 12 hydrogen atoms right here, and then this part over here says that there are 6 oxygen atoms. So we can fill that in right here.
And so, this here is what we refer to as a chemical formula and you'll be able to see many more examples of these as we make our way through our course. Another example would be H2O is another example of a chemical formula. But this here concludes our introduction to chemical bonds and how they form molecules and compounds and moving forward we're going to talk about many different types of chemical bonds. So I'll see you all in our next video.