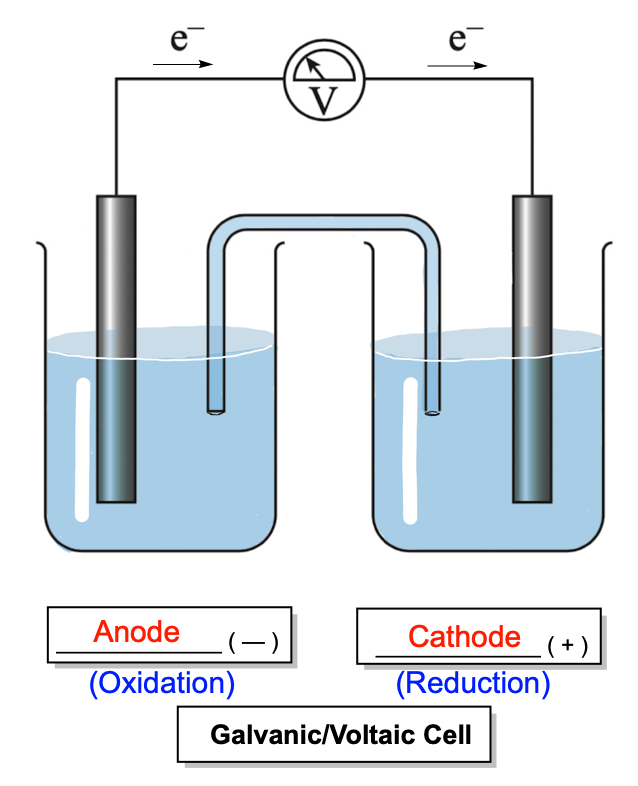
Hey everyone. So, galvanic or voltaic cells are spontaneous cells that produce or discharge electricity. Now remember, when they discharge all their electricity, then they're classified as a dead battery. Now, here we have a basic model of a galvanic cell, voltaic cell. And we're going to say that in a galvanic or voltaic cell, the negative electrode is the anode and the positive electrode is the cathode.
Here we have 2 half reactions that are designated for the cathode and the anode. For the cathode, we have 3 moles of copper 2 ion absorbing 6 moles of electrons to produce 3 moles of copper solid. So here that means we'd have Cu2+ particles floating around within the solution. The metal electrode itself is copper solid. For the anode half reaction, we have 2 moles of chromium solid, basically losing 6 electrons to produce 2 moles of chromium 3 ions.
So in this compartment here, we have our chromium solid here, and we have Cr3+ positive ions floating around. Now with the galvanic or voltaic cell, we're going to say that these 2 jars are connected by this, which is called a salt bridge. Now, in a salt bridge, we have neutral ions, ions that are neither acidic nor basic in nature. Typically, we have chloride ions or nitrate ions. Those negative ions, they flow toward the anode side, and then we may have sodium ions and potassium ions which move this way towards the cathode side.
We say that oxidation always occurs at the anode, and reduction always occurs at the cathode. That's why the anode is losing electrons. Electrons move away from the anode towards the cathode. As they move we have the traveling of electrons from one side to another, and in order to close this circuit, we need the same charges moving in the opposite direction. So we have our anode here, we have our cathode here, we have electrons moving toward the cathode, and within the salt bridge, we have our negative ions moving towards the anode side.
This completes the circuit, and so through the movement of these 2 electrons, of these 2 charge-like charges in opposite directions, we form a current. So this voltmeter here reads how much voltage is being produced. Now, we want our anodes to lose electrons easily, so we want their ionization energy, the energy required to move an electron, to be low. We want the cathode to attract the electrons, so we want its electron affinity to be high. What starts to happen is you're losing electrons over time, so this anode electrons weigh very little, but enough of them over time you start losing some mass.
So we're going to say here your anode dissolves away. As the cathode gains more and more electrons, its surface starts to become negatively charged. This attracts the submerged or dissolved copper ions within the solution. So you have positive ions adhering to a surface that's slightly negative in charge, they're going to neutralize each other, and so you're going to have an encrusting of copper on top of this electrode. So over time, the cathode is going to get bigger and we say the cathode plates out.
Now to produce this electricity you want to make sure that the cathode side is attracting these negative electrons, so you want to make sure that this solution here has a good amount of positive charges in it that's going to attract the negative electrons toward that side. You want to make sure the cathode solution concentration is high. At the same time, you want to make sure that the anode concentration of positive ions is low. Because if this solution becomes too saturated with positive ions those negative electrons will not want to move towards the cathode. Because remember, like charges attract each other.
So this gives us the basic breakdown of our galvanic or voltaic cell. Remember with the anode we have oxidation and with the cathode we have reduction. We have 2 half reactions involved within this particular example, but we also have other examples of half half reactions. All of them are written as reductions because for all of them the electrons are reactants. With each one we have a standard cell potential associated with it.
Remember that the higher your standard cell potential, then the more likely reduction will occur. And remember, if the more likely reduction occurs that means you have a stronger oxidizing agent. Conversely, the lower your standard cell potential is, then the more likely oxidation will occur. So more likely is oxidation, and the more likely oxidation happens the stronger your reducing agent. Okay.
So just remember for this particular example we're looking at 2 specific half reactions, but we could easily come up with another galvanic or voltaic cell example which incorporates some of these other half reduction reactions.