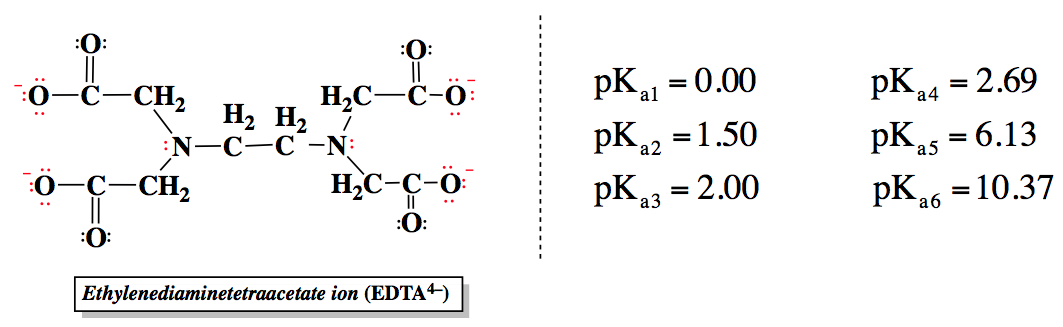
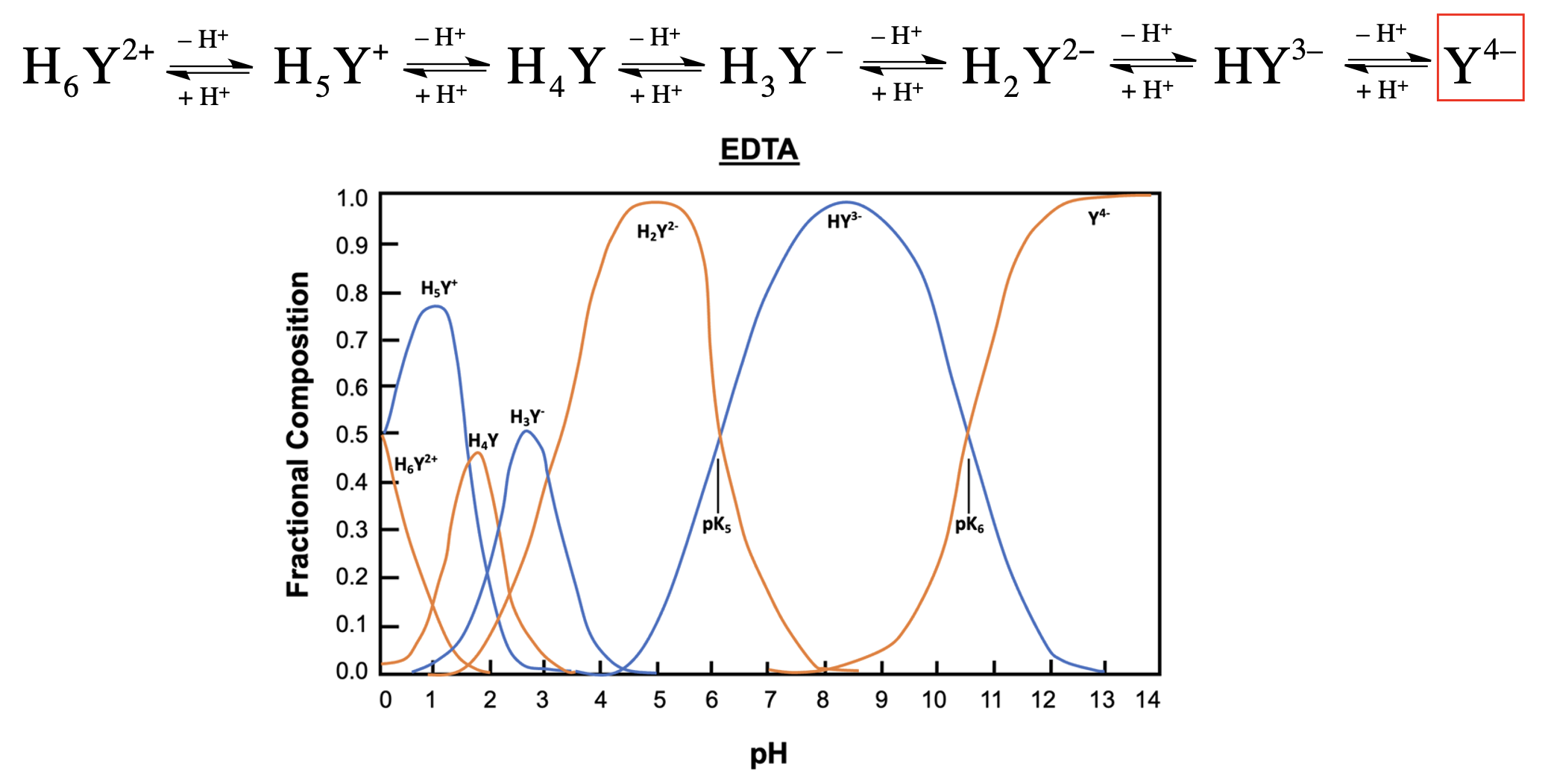
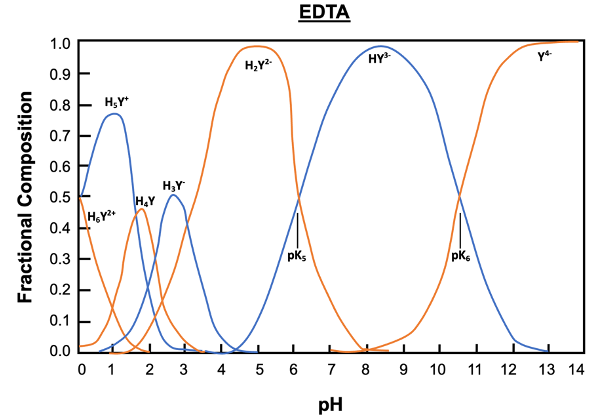
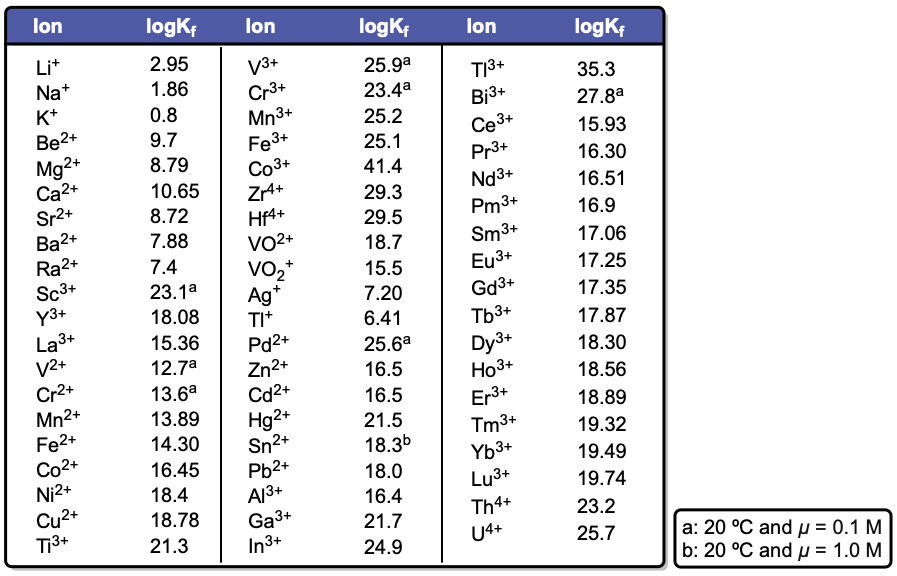
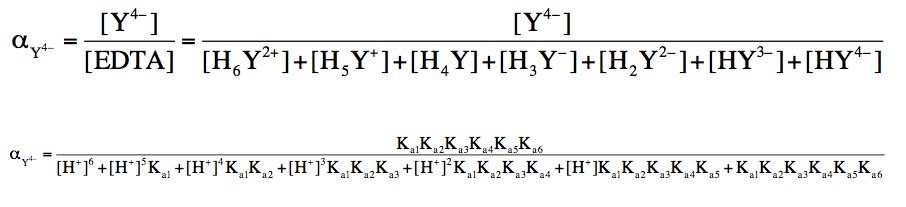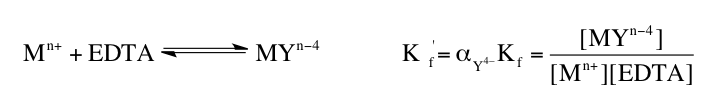EDTA, or ethylenediaminetetraacetic acid, is a versatile compound that can exist in multiple protonation states, depending on the pH of the solution. In its fully protonated form, EDTA is a hexaprotic acid, represented as H6Y2+, indicating it can donate up to six protons. As the pH increases, protons are removed, leading to various deprotonated forms, with the fully deprotonated state being EDTA4-.
EDTA contains four carboxylate groups and two amine groups, each contributing to its acidity and pKa values. Carboxylic acids are generally strong organic acids, with lower pKa values, while the amine groups have higher pKa values. The relationship between pH and pKa is crucial: if the pH is greater than the pKa, deprotonation occurs; if the pH is less, protonation takes place. This dynamic allows EDTA to adapt its charge based on the surrounding environment.
In total, EDTA can exist in up to seven different forms, influenced by the solution's pH. The fully protonated form is stable in acidic conditions, while the neutral form is preferred for long-term transport. The basic form, where all acidic protons are removed, is optimal for forming metal complexes. A graph illustrating the predominance of these forms at various pH levels shows that at a pH of 6, there is an equilibrium between two specific forms of EDTA, while at a pH above 10.37, the basic form becomes dominant.
To calculate the fraction of EDTA in its basic form, the following equation is used:
Fraction of Basic Form = \(\frac{[Basic \, Form]}{[EDTA]}\)
Here, [EDTA] refers to the total concentration of all seven forms. This equation can be further refined when the pH is known, as the concentration of hydrogen ions \([H^+]\) can be derived from the pH using the formula:
\([H^+] = 10^{-\text{pH}}\)
Additionally, the acid dissociation constant \(K_a\) is related to pKa by the equation:
\(K_a = 10^{-\text{pKa}}\)
Understanding the various forms of EDTA and their dependence on pH is essential for effectively utilizing this compound in applications, particularly in metal complexation, where the basic form is most advantageous.