Basic Chemistry, 6th Edition © 2020
Karen C. Timberlake | William Timberlake

Basic Chemistry prepares students for science-related professions, such as engineering, nursing, medicine, environmental or agricultural science, and laboratory technology. The authors make the study of chemistry an engaging and a positive experience by relating the structure and behavior of matter to real life. The goal of the text is to help students become critical thinkers by understanding scientific concepts.
The 6th edition presents more challenging problems and new practice problems to help students master the basic quantitative skills and conceptual understanding for success in this course.
Hallmark features of this title
- Concept checks encourage students to revisit their understanding of key terms and ideas before moving forward.
- Understanding the concepts are questions with visual representations that build an understanding of newly learned chemical concepts.
- Marginal notes, end-of-chapter problems, and an expanded media program deepen the connection between key math skills and why they are so important to success in the course.
- Macro-to-micro art illustrations enable students to make connections between recognizable objects and their atomic-level representations to help them visualize chemistry in everyday life.
- Chemistry links to health and chemistry links to the environment relate chemistry concepts to real-life topics in health, the environment, and medicine.
New and updated features of this title
- Practice problems appear in the margin, showing students which practice problems align with the content and sample problems throughout the text.
- Additional practice problems and challenge problems test student understanding of the topics in the chapter.
- Updated: Connect feature is now part of analyze the problem boxes. These specify information related to the given and need sections to help students identify and connect the components within a word problem and set up a solution strategy.
- Expanded: Study check questions within each sample problem help students review problem-solving strategies and their comprehension of the material.
- Updated: Engage questions include answers at the end of the chapter and reflect research on the way students learn and retain information to help students associate new content with knowledge in their long-term memory.
- Updated: Chapter openers provide timely examples and engaging, topical issues of chemistry that are part of contemporary professions.
- Updated: Interactive videos give students an opportunity to reinforce what they just learned by showing how chemistry works in real life and introducing a bit of humor into chemical problem solving and demonstrations.
- New & updated: Enhanced end-of-chapter questions with answer-specific feedback guide students towards the correct answer without giving the answer away.
- Key math skills and core chemistry skills tutorials provide assignable practice problems related to the in-text feature boxes, ensuring that students master the basic quantitative and science skills they need to succeed in the course.
- The Chemistry Primer helps students remediate chemistry math skills and prepare for their first college chemistry course. Pre-built assignments can be assigned before the course begins or as just-in-time remediation to ensure students practice and maintain their math skills.
1. CHEMISTRY IN OUR LIVES
1.1 Chemistry and Chemicals
1.2 Scientific Method: Thinking Like a Scientist
1.3 Studying and Learning Chemistry
1.4 Key Math Skills for Chemistry
1.5 Writing Numbers in Scientific Notation
2. CHEMISTRY AND MEASUREMENTS
2.1 Units of Measurement
2.2 Measured Numbers and Significant Figures
2.3 Significant Figures in Calculations
2.4 Prefixes and Equalities
2.5 Writing Conversion Factors
2.6 Problem Solving Using Unit Conversion
2.7 Density
3. MATTER AND ENERGY
3.1 Classification of Matter
3.2 States and Properties of Matter
3.3 Temperature
3.4 Energy
3.5 Specific Heat
3.6 Energy and Nutrition
4. ATOMS AND ELEMENTS
4.1 Elements and Symbols
4.2 The Periodic Table
4.3 The Atom
4.4 Atomic Number and Mass Number
4.5 Isotopes and Atomic Mass
5. ELECTRONIC STRUCTURE OF ATOMS AND PERIODIC TRENDS
5.1 Electromagnetic Radiation
5.2 Atomic Spectra and Energy Levels
5.3 Sublevels and Orbitals
5.4 Orbital Diagrams and Electron Configurations
5.5 Electron Configurations and the Periodic Table
5.6 Trends in Periodic Properties
6. IONIC AND MOLECULAR COMPOUNDS
6.1 Ions: Transfer of Electrons
6.2 Ionic Compounds
6.3 Naming and Writing Ionic Formulas
6.4 Polyatomic Ions
6.5 Molecular Compounds: Sharing Electrons
7. CHEMICAL QUANTITIES
7.1 The Mole
7.2 Molar Mass
7.3 Calculations Using Molar Mass
7.4 Mass Percent Composition
7.5 Empirical Formulas
7.6 Molecular Formulas
8. CHEMICAL REACTIONS
8.1 Equations for Chemical Reactions
8.2 Balancing a Chemical Equation
8.3 Types of Chemical Reactions
8.4 Oxidation—Reduction Reactions
9. CHEMICAL QUANTITIES IN REACTIONS
9.1 Conservation of Mass
9.2 Mole Relationships in Chemical Equations
9.3 Mass Calculations for Chemical Reactions
9.4 Limiting Reactants
9.5 Percent Yield
9.6 Energy in Chemical Reactions
10. BONDING AND PROPERTIES OF SOLIDS AND LIQUIDS
10.1 Lewis Structures for Molecules and Polyatomic Ions
10.2 Resonance Structures
10.3 Shapes of Molecules and Polyatomic Ions (VSEPR Theory)
10.4 Electronegativity and Bond Polarity
10.5 Polarity of Molecules
10.6 Intermolecular Forces Between Atoms or Molecules
10.7 Changes of State
11. GASES
11.1 Properties of Gases
11.2 Pressure and Volume (Boyle’s Law)
11.3 Temperature and Volume (Charles’s Law)
11.4 Temperature and Pressure (Gay-Lussac’s Law)
11.5 The Combined Gas Law
11.6 Volume and Moles (Avogadro’s Law)
11.7 The Ideal Gas Law
11.8 Gas Laws and Chemical Reactions
11.9 Partial Pressures (Dalton’s Law)
12. SOLUTIONS
12.1 Solutions
12.2 Electrolytes and Nonelectrolytes
12.3 Solubility
12.4 Solution Concentrations
12.5 Dilution of Solutions
12.6 Chemical Reactions in Solution
12.7 Molality and Freezing Point Lowering/Boiling Point Elevation
12.8 Properties of Solutions: Osmosis
13. REACTION RATES AND CHEMICAL EQUILIBRIUM
13.1 Rates of Reactions
13.2 Chemical Equilibrium
13.3 Equilibrium Constants
13.4 Using Equilibrium Constants
13.5 Changing Equilibrium Conditions: Le Châtelier’s Principle
14. ACIDS AND BASES
14.1 Acids and Bases
14.2 Brønsted—Lowry Acids and Bases
14.3 Strengths of Acids and Bases
14.4 Dissociation Constants of Weak Acids and Bases
14.5 Dissociation of Water
14.6 The pH Scale
14.7 Reactions of Acids and Bases
14.8 Acid—Base Titration
14.9 Buffers
15. OXIDATION AND REDUCTION
15.1 Oxidation and Reduction
15.2 Balancing Oxidation—Reduction Equations Using Half-Reactions
15.3 Electrical Energy from Oxidation—Reduction Reactions
15.4 Oxidation—Reduction Reactions That Require Electrical Energy
16. NUCLEAR CHEMISTRY
16.1 Natural Radioactivity
16.2 Nuclear Reactions
16.3 Radiation Measurement
16.4 Half-Life of a Radioisotope
16.5 Medical Applications Using Radioactivity
16.6 Nuclear Fission and Fusion
17. ORGANIC CHEMISTRY
17.1 Alkanes
17.2 Alkenes, Alkynes, and Polymers
17.3 Aromatic Compounds
17.4 Alcohols and Ethers
17.5 Aldehydes and Ketones
17.6 Carboxylic Acids and Esters
17.7 Amines and Amides
18. BIOCHEMISTRY
18.1 Carbohydrates
18.2 Disaccharides and Polysaccharides
18.3 Lipids
18.4 Amino Acids and Proteins
18.5 Protein Structure
18.6 Proteins as Enzymes
18.7 Nucleic Acids
18.8 Protein Synthesis
Karen C. Timberlake
Los Angeles Valley College
Karen Timberlake is Professor Emerita of Chemistry at Los Angeles Valley College, where she taught chemistry for allied health and preparatory chemistry for 36 years. She received her bachelor's degree in chemistry from the University of Washington and her master's degree in biochemistry from the University of California at Los Angeles.
Professor Timberlake has been writing chemistry textbooks for more than 40 years. During that time, her name has become associated with the strategic use of pedagogical tools that promote student success in chemistry and the application of chemistry to real-life situations. More than one million students have learned chemistry using texts, laboratory manuals, and study guides written by Karen Timberlake. In addition to Basic Chemistry, 6th edition, she is also the author of General, Organic, and Biological Chemistry: Structures of Life, 6th edition, with the accompanying study guide, and Chemistry: An Introduction to General, Organic, and Biological Chemistry, 13th edition, with the accompanying study guide and Selected Solutions Manual, Laboratory Manual, and Essential Laboratory Manual.
Professor Timberlake belongs to numerous scientific and educational organizations including the American Chemical Society (ACS) and the National Science Teachers Association (NSTA). She has been the Western Regional Winner of Excellence in College Chemistry Teaching Award given by the Chemical Manufacturers Association. She received the McGuffey Award in Physical Sciences from the Textbook Authors Association for her textbook Chemistry: An Introduction to General, Organic, and Biological Chemistry, 8th Edition. She received the “Texty” Textbook Excellence Award from the Textbook Authors Association for the 1st edition of Basic Chemistry. She has participated in education grants for science teaching including the Los Angeles Collaborative for Teaching Excellence (LACTE) and a Title III grant at her college. She speaks at conferences and educational meetings on the use of student-centered teaching methods in chemistry to promote the learning success of students. When Professor Timberlake is not writing textbooks, she relaxes by playing tennis, ballroom dancing, hiking, traveling, trying new restaurants, cooking, and enjoying her grandchildren, Daniel and Emily.
William Timberlake
Los Angeles Valley College
William Timberlake, husband of Karen C. Timberlake, who is the coauthor of this program, is Professor Emeritus of Chemistry at Los Angeles Harbor College, where he taught preparatory and organic chemistry for 36 years. He received his bachelor's degree in chemistry from Carnegie Mellon University and his master's degree in organic chemistry from the University of California at Los Angeles. When Professor Timberlake is not writing textbooks, he relaxes by playing tennis, ballroom dancing, hiking, traveling, trying new restaurants, cooking, and enjoying his grandchildren, Daniel and Emily.
This program is available in a variety of formats. You can review the individual prices for each ISBN in our catalog. All access codes are for use by 1 student, for 1 course, for up to 1 year, and are non-transferable.
| Format | ISBN-13 |
|---|---|
| Student edition (HS binding) with Modified Mastering Chemistry with Pearson eTextbook (up to 6-years) | 9780135244616 |
| Modified Mastering Chemistry with Pearson eTextbook (1-year access) | 9780137452392 |
| Modified Mastering Chemistry with Pearson eTextbook (6-year access) | 9780137452460 |

Not seeing what you’re looking for?
Check out our catalog
Review all our AP®, Honors, and Electives programs, including available formats, prices, and ISBNs
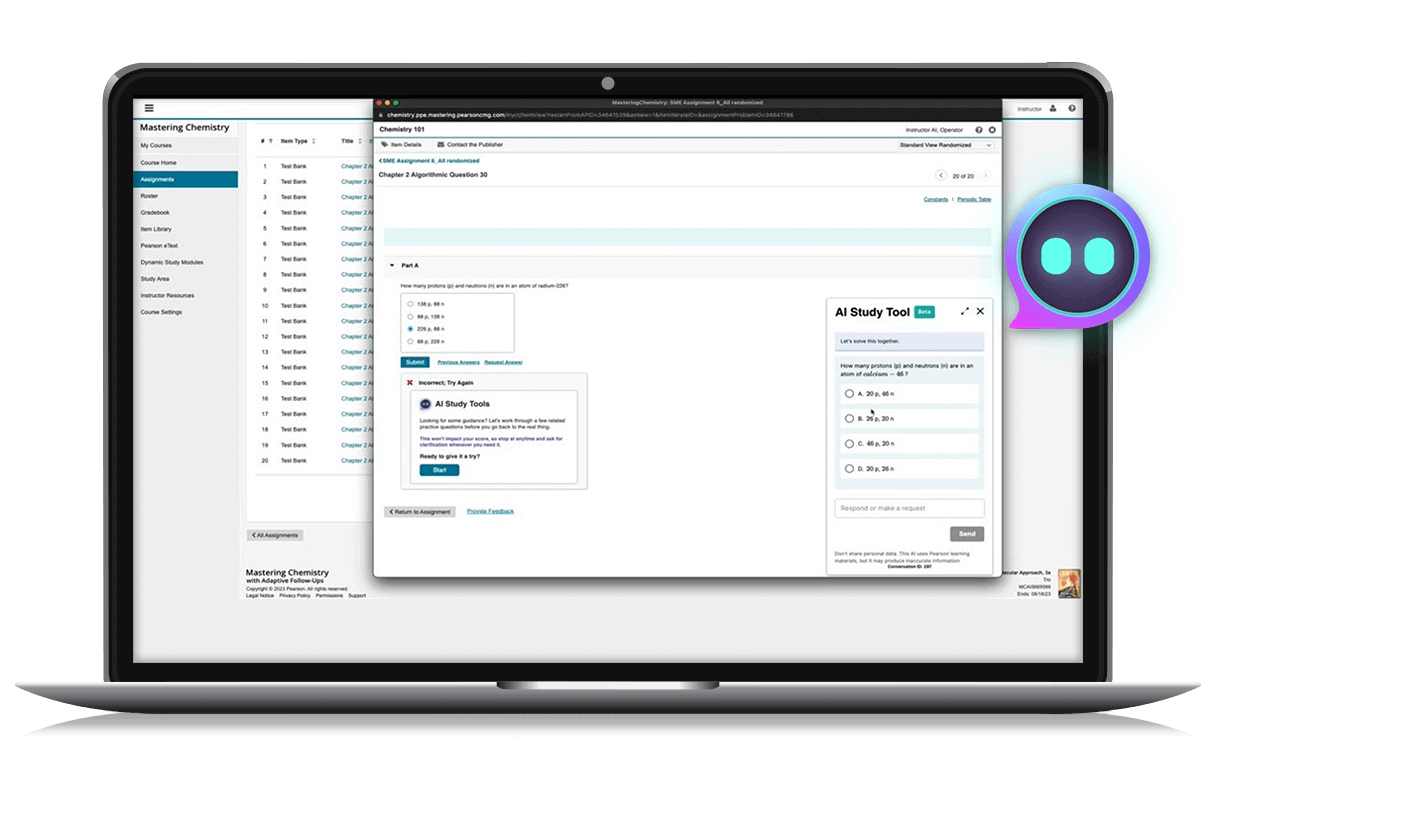
Online learning
Mastering
An immersive and adaptable online learning platform that empowers students to learn through active and engaging experiences. With personalized tutorials, analytics, and feedback, Mastering® helps high school students develop a strong foundation in science and engineering programs.
If you'd like to see a demo of Mastering, contact our team.

AP®, Honors, & Electives Catalog
Not seeing what you’re looking for?
Review all our AP®, Honors, and Electives programs, including available formats, prices, and ISBNs
Contact Pearson
Our experts will guide you through available programs, eTextbooks, and printed materials.
With this form, you can request a sample, access instructor resources, or make a purchase.
AP® is a trademark registered and/or owned by the College Board, which was not involved in the production of, and does not endorse, these programs.