Hey, guys. So we've solved lots of problems with PV diagrams. But one of the most important and common ones that you'll see is one where you start off at some position in the PV diagram. You'll do a sequence of steps and then you'll end right back where you started. It basically just goes in a loop like this. These are called cyclic thermodynamic processes, and there are a couple of really important properties that you need to know about them. In this video, we're going to dive right in. We're going to solve this problem together, and let's go ahead and check it out.
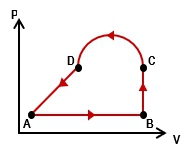
As a cyclic process is one where a system or gas completes a sequence of steps but returns to its initial state. So that's what a cycle is. It returns back to the initial states. There are 2 important properties here. Rather than tell you, I want to show you. So let's just go ahead and take a look at our problem here. So we have this ideal gas and it goes through this process. We want to calculate the work that's done by the gas over the cycle, so the total amount of work done. So how do we do that? Well, let's see.
In part a, if we want to calculate the total amount of work done remember, this is just a bunch of processes sort of strung together. The way we calculate the net work or the total work is you just have to add up all the works done by all these individual processes. Right? We've done that before. So, basically, if I have from a to b and b to c and so on and so forth, the total work will just be the work done across all of these different steps. So the work done from a to b, b to c, c to d, and then the work done from d back to a.
It might seem there are a lot of terms and we're going to have to go calculate a lot of stuff. But let's just go ahead and refer to our table here to make some of these things really easy. So if you think about this sort of square here or rectangle is really made up of 2 different types of steps, isobaric and isovolumetric. What's easy about that is we have the work equations for both of these. It turns out what happens is, remember, in isovolumetric processes, the work done is equal to 0. So that means from a to b, there is no work done because there's no change in volume. Right? And then from c to d, this is also a vertical line, so there's no work done here.
Really, instead of 4 times, we only have 2. These two are isobaric processes, which means we'll be able to use p delta v. So, that just means we're going to take this work equation and it simplifies to the work done from b to c. That's going to be the pressure at b. It's the pressure here. So this is \( p_b \times \Delta v \), from b to c plus the work the pressure done at \( p \), the pressure done here at d. Sorry. That's \( p_d \) here, and then times the change in the volume from d back to a.
So let's take a look at this. Basically, I'm just going to start plugging in some numbers here. \( p_b \) is 35. What's the change in volume from b to c? We're going from 28 to 40 here. So what that means here is that this \( \Delta v \) is equal to 12 from here to here. Alright? So, \( 28 \), you know, the difference between 28 and 40. This is going to be 12. And then what about actually, let us go ahead and plug it in, you know, \( 40 - 28 \) to do final minus initial. So then what about this \( p_d \) here? This is going to be 15, and then the change in the volume here, now from d back to a, remember that this arrow here goes to the left. So my final minus initial is actually \( 28 - 40 \). So it's going to be \( 28 - 40 \).
So, if you go ahead and work this out here, what you're going to end up getting is that the total amount of work done over this entire cycle is 240 joules. Alright? So that's the answer. Let's move on to part b now. In part b, we want to calculate the area enclosed within the loop. The area enclosed is going to be this area that's sort of enclosed insideacji