First Law of Thermodynamics definitions Flashcards
 Back
BackFirst Law of Thermodynamics definitions
1/15
Terms in this set (15)
- Internal EnergyThe total energy contained within a system, influenced by heat and work.
- HeatA form of energy transfer to or from a system, affecting its internal energy.
- WorkEnergy transfer resulting from a force applied over a distance, affecting a system's energy.
- SystemA defined portion of the universe under study, often a gas in thermodynamics.
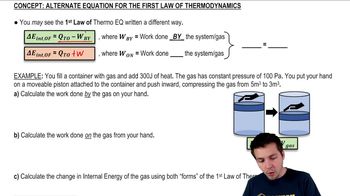
- First Law of ThermodynamicsA principle stating that the change in internal energy equals heat added minus work done by the system.
- PressureThe force exerted per unit area within a system, crucial for calculating work.
- VolumeThe space occupied by a system, changes in which affect work done.
- Sign ConventionRules determining the positive or negative values of heat and work in thermodynamic equations.
- PistonA movable component in a system that can change volume, affecting work and energy.
- Constant PressureA condition where pressure remains unchanged, simplifying work calculations.
- Delta ESymbol representing the change in internal energy of a system.
- QSymbol representing the heat added to or removed from a system.
- WSymbol representing the work done by or on a system.
- Thermal EnergyEnergy related to the temperature of a system, transferred as heat.
- Mechanical EnergyEnergy associated with the motion or position of an object, transferred as work.