Entropy Equations for Special Processes definitions Flashcards
 Back
BackEntropy Equations for Special Processes definitions
1/15
Terms in this set (15)
- EntropyA measure of the disorder or randomness in a system, often associated with the amount of energy unavailable for doing work.
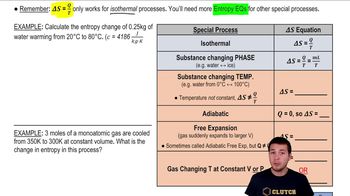
- Isothermal ProcessA thermodynamic process that occurs at a constant temperature, where Delta S = Q/T applies.
- Phase ChangeA transition of matter from one state to another, such as solid to liquid, where temperature remains constant.
- Adiabatic ProcessA process with no heat transfer into or out of the system, resulting in Delta S = 0.
- Free ExpansionA process where a gas expands into a larger volume without external work, increasing entropy.
- Isochoric ProcessA thermodynamic process at constant volume, where entropy change is calculated using n*Cv*Ln(T_final/T_initial).
- Isobaric ProcessA thermodynamic process at constant pressure, where entropy change is calculated using n*Cp*Ln(T_final/T_initial).
- Heat CapacityThe amount of heat required to change a substance's temperature by a given amount, denoted as Cv or Cp.
- Natural LogarithmA logarithm to the base e, used in entropy equations to express ratios of final to initial states.
- Specific HeatThe heat required to raise the temperature of a unit mass of a substance by one degree Celsius.
- Universal Gas ConstantA constant denoted as R, used in equations involving gases, approximately 8.314 J/(mol·K).
- Monoatomic GasA gas consisting of single atoms, often with specific heat capacities like Cv = 3/2 R.
- CalorimetryThe science of measuring the heat of chemical reactions or physical changes.
- Delta SSymbol representing the change in entropy of a system during a process.
- MolesA unit of measurement for amount of substance, used in thermodynamic equations.