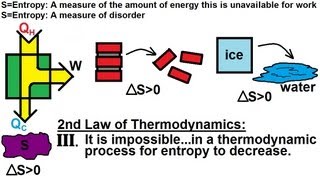
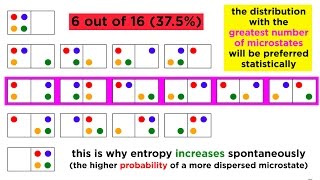
23. The Second Law of Thermodynamics
Entropy and the Second Law of Thermodynamics
23. The Second Law of Thermodynamics
Entropy and the Second Law of Thermodynamics
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
3 moles of an ideal gas are compressed isothermally at 20°C. During this compression, 1850 J of work is done on the gas. What is the change of entropy of the gas?
766views1rank1comments - Multiple Choice
You have a block of ice at 0°C. Heat is added to the ice, causing an increase in entropy of 120J/K. How much ice melts into water in this process?
550views3rank - Multiple Choice
A non-Carnot heat engine operates between a hot reservoir at 610K and a cold reservoir at 320K. In a cycle, it takes in 6400 J of heat and does 2200 J of work. What is the total change in entropy of the universe over the cycle?
470views1rank - Textbook QuestionCALC Two moles of an ideal gas occupy a volume V. The gas expands isothermally and reversibly to a volume 3V. (a) Is the velocity distribution changed by the isothermal expansion? Explain.641views
- Textbook QuestionCALC You make tea with 0.250 kg of 85.0°C water and let it cool to room temperature 120.0°C2. (a) Calculate the entropy change of the water while it cools.440views
- Textbook QuestionA 15.0-kg block of ice at 0.0°C melts to liquid water at 0.0°C inside a large room at 20.0°C. Treat the ice and the room as an isolated system, and assume that the room is large enough for its temperature change to be ignored. (a) Is the melting of the ice reversible or irreversible? Explain, using simple physical reasoning without resorting to any equations.663views
- Textbook QuestionCALC You decide to take a nice hot bath but discover that your thoughtless roommate has used up most of the hot water. You fill the tub with 195 kg of 30.0°C water and attempt to warm it further by pouring in 5.00 kg of boiling water from the stove. (a) Is this a reversible or an irreversible process? Use physical reasoning to explain.591views
- Multiple ChoiceWhich of the following statements is consistent with the Second Law of Thermodynamics?3views