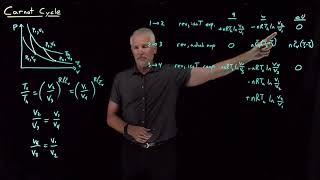23. The Second Law of Thermodynamics
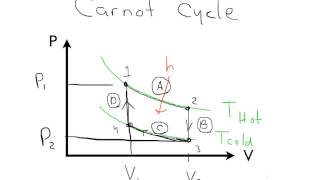
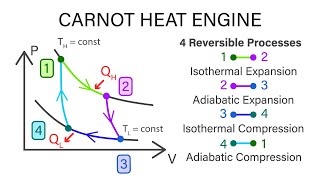
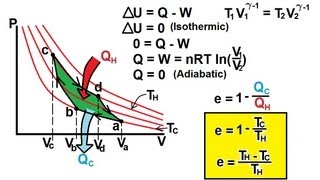
The Carnot Cycle
23. The Second Law of Thermodynamics
The Carnot Cycle
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
A theoretical heat engine in space could operate between the Sun's 5500°C surface and the –270.3°C temperature of intergalactic space. What would be its maximum theoretical efficiency?
475views2rank1comments - Multiple Choice
A Carnot engine with an efficiency of 70% is cooled by water at 10°C. What temperature must the hot reservoir be maintained at?
1275views1rank - Multiple Choice
Your friend claims they have a design for a reversible heat engine that can operate between the freezing and boiling temperatures of water that has an efficiency of 30%. Is this possible?
459views - Multiple ChoiceWhat is the Carnot efficiency of a heat engine operating between a hot reservoir at 300°C and a cold reservoir at 10°C?398views
- Textbook QuestionA Carnot heat engine uses a hot reservoir consisting of a large amount of boiling water and a cold reservoir consisting of a large tub of ice and water. In 5 minutes of operation, the heat rejected by the engine melts 0.0400 kg of ice. During this time, how much work W is performed by the engine?1262views
- Textbook QuestionA Carnot engine is operated between two heat reservoirs at temperatures of 520 K and 300 K. (c) What is the thermal efficiency of the engine?408views
- Textbook QuestionA Carnot engine is operated between two heat reservoirs at temperatures of 520 K and 300 K. (b) How much mechanical work is performed by the engine during each cycle?735views
- Textbook QuestionA Carnot engine is operated between two heat reservoirs at temperatures of 520 K and 300 K. (a) If the engine receives 6.45 kJ of heat energy from the reservoir at 520 K in each cycle, how many joules per cycle does it discard to the reservoir at 300 K?1544views