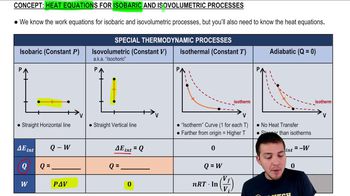
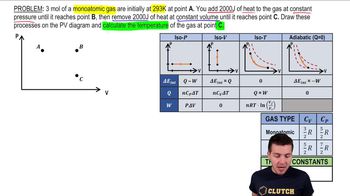
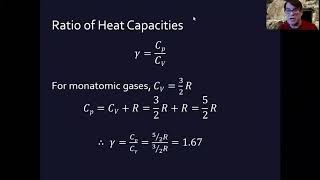
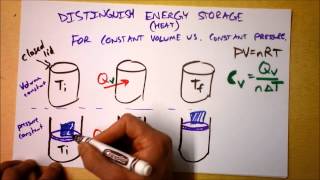
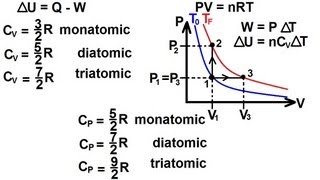
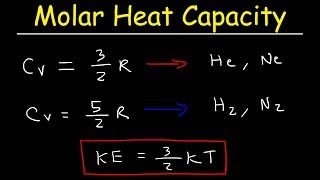
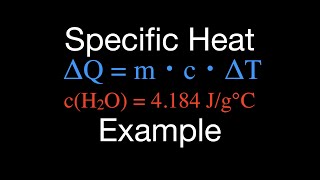
22. The First Law of Thermodynamics
Heat Equations for Special Processes & Molar Specific Heats
22. The First Law of Thermodynamics
Heat Equations for Special Processes & Molar Specific Heats
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
How much heat energy is needed to increase the temperature of 5 mol of an ideal diatomic gas by from 273K to 300K if the a) pressure is held constant; b) the volume is held constant?
541views4rank - Textbook QuestionCALC The temperature of 0.150 mol of an ideal gas is held constant at 77.0°C while its volume is reduced to 25.0% of its initial volume. The initial pressure of the gas is 1.25 atm. (c) Does the gas exchange heat with its surroundings? If so, how much? Does the gas absorb or liberate heat?575views
- Textbook QuestionA cylinder contains 0.100 mol of an ideal monatomic gas. Initially the gas is at 1.00 * 10^5 Pa and occupies a volume of 2.50 * 10^-3 m^3. (b) If the gas is allowed to expand to twice the initial volume, find the final temperature (in kelvins) and pressure of the gas if the expansion is (i) isothermal; (ii) isobaric; (iii) adiabatic.526views
- Textbook QuestionA cylinder contains 0.100 mol of an ideal monatomic gas. Initially the gas is at 1.00 * 10^5 Pa and occupies a volume of 2.50 * 10^-3 m^3. (b) If the gas is allowed to expand to twice the initial volume, find the final temperature (in kelvins) and pressure of the gas if the expansion is (i) isothermal; (ii) isobaric; (iii) adiabatic.799views
- Textbook QuestionA cylinder contains 0.100 mol of an ideal monatomic gas. Initially the gas is at 1.00 * 10^5 Pa and occupies a volume of 2.50 * 10^-3 m^3. (b) If the gas is allowed to expand to twice the initial volume, find the final temperature (in kelvins) and pressure of the gas if the expansion is (i) isothermal; (ii) isobaric; (iii) adiabatic.437views
- Multiple ChoiceAdiabatic temperature changes result from which of the following conditions in the atmosphere?6views