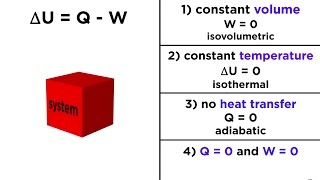
22. The First Law of Thermodynamics
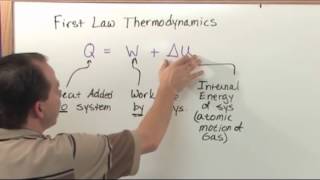
First Law of Thermodynamics
Learn with other creators
Practice this topic
- Multiple Choice
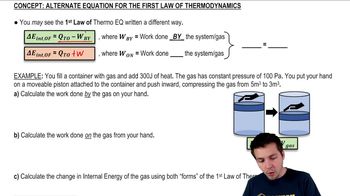
A gas in a cylinder held at a constant pressure 1.80×105 Pa expands from a volume of 1.2 m3 to 1.6 m3. The internal energy of the gas decreases from 4.40×105 J to 3×105 J. How much heat was transferred to the gas?
790views10rank - Multiple Choice
The internal energy of a system decreases by 500 J, and 230 J of work is done on the system. What is the heat transfer into or out of this system?
746views10rank - Textbook Question
Boiling Water at High Pressure. When water is boiled at a pressure of 2.00 atm, the heat of vaporization is 2.20 * 10^6 J/kg and the boiling point is 120°C. At this pressure, 1.00 kg of water has a volume of 1.00 * 10^-3 m^3 , and 1.00 kg of steam has a volume of 0.824 m^3. (b) Compute the increase in internal energy of the water.
540views - Textbook Question
A gas in a cylinder is held at a constant pressure of 1.80 * 10^5 Pa and is cooled and compressed from 1.70 m^3 to 1.20 m^3. The internal energy of the gas decreases by 1.40 * 10^5 J. (c) Does it matter whether the gas is ideal? Why or why not?
518views - Textbook Question
Five moles of an ideal monatomic gas with an initial temperature of 127°C expand and, in the process, absorb 1500 J of heat and do 2100 J of work. What is the final temperature of the gas?
987views - Textbook Question
Boiling Water at High Pressure. When water is boiled at a pressure of 2.00 atm, the heat of vaporization is 2.20 * 10^6 J/kg and the boiling point is 120°C. At this pressure, 1.00 kg of water has a volume of 1.00 * 10^-3 m^3 , and 1.00 kg of steam has a volume of 0.824 m^3. (a) Compute the work done when 1.00 kg of steam is formed at this temperature.
2541views - Multiple ChoiceWhich statement regarding a closed system is accurate according to the First Law of Thermodynamics?25views
- Multiple ChoiceWhich of the following statements describes the first law of thermodynamics?32views