20. Heat and Temperature
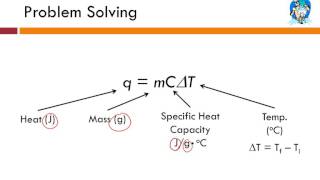
Specific Heat & Temperature Changes
20. Heat and Temperature
Specific Heat & Temperature Changes
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
You are given a sample of an unknown metal. You weigh the sample and find that its weight is 29.4N. You add 1.25×104 J of heat energy to the sample and find that its temperature increases from 52°C to 70°C. What is the specific heat of this unknown metal?
611views8rank - Multiple Choiceof heat energy are added to a block of copper that is initially at . What is the final temperature of the copper? The specific heat capacity of copper is .371views
- Textbook QuestionA nail driven into a board increases in temperature. If we assume that 60% of the kinetic energy delivered by a 1.80-kg hammer with a speed of 7.80 m/s is transformed into heat that flows into the nail and does not flow out, what is the temperature increase of an 8.00-g aluminum nail after it is struck ten times?1088views1comments
- Textbook QuestionWhile painting the top of an antenna 225 m in height, a worker accidentally lets a 1.00-L water bottle fall from his lunchbox. The bottle lands in some bushes at ground level and does not break. If a quantity of heat equal to the magnitude of the change in mechanical energy of the water goes into the water, what is its increase in temperature?832views
- Textbook QuestionIn an effort to stay awake for an all-night study session, a student makes a cup of coffee by first placing a 200-W electric immersion heater in 0.320 kg of water. (b) How much time is required? Assume that all of the heater's power goes into heating the water612views
- Textbook QuestionIn very cold weather a significant mechanism for heat loss by the human body is energy expended in warming the air taken into the lungs with each breath. (a) On a cold winter day when the temperature is -20°C, what amount of heat is needed to warm to body temperature (37°C) the 0.50 L of air exchanged with each breath? Assume that the specific heat of air is 1020 J/kg K and that 1.0 L of air has mass 1.3 * 10^-3 kg.1232views
- Multiple ChoiceWhich of the following phenomena can be attributed to water's high specific heat capacity?3views
- Multiple ChoiceHow does the specific heat capacity of water affect the climates of coastal regions?5views