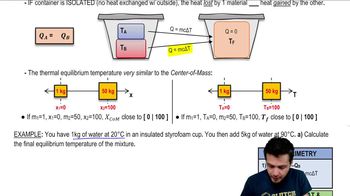
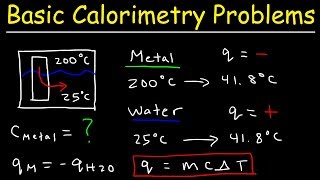
20. Heat and Temperature
Intro to Calorimetry
20. Heat and Temperature
Intro to Calorimetry
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Textbook QuestionYou have 750 g of water at 10.0°C in a large insulated beaker. How much boiling water at 100.0°C must you add to this beaker so that the final temperature of the mixture will be 75°C?1499views
- Textbook QuestionA 750 g aluminum pan is removed from the stove and plunged into a sink filled with 10.0 L of water at 20.0°C . The water temperature quickly rises to 24.0°C. What was the initial temperature of the pan in °C and in °F?450views
- Textbook Question
(II) A 215-g sample of a substance is heated to 330°C and then plunged into a 105-g aluminum calorimeter cup containing 185 g of water and a 17-g glass thermometer at 10.5°C. The final temperature is 35.0°C. What is the specific heat of the substance? (Assume no water boils away.)
173views - Textbook Question30 g of copper pellets are removed from a 300°C oven and immediately dropped into 100 mL of water at 20°C in an insulated cup. What will the new water temperature be?596views