- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

A mass of 0.300 kg of water at 60.0° C is placed in a massless thermos bottle. How many grams of ice cubes, at a temperature of -10.0°C, must be poured into the water to get the system's final temperature to 30.0°C?
In an experiment, a student releases 10 pieces of one-dollar coins heated to 140 °C into a thermally insulating container of mass 50 g and specific heat capacity 420 J/kg•°C. The container is filled with a mixture of ice (5 g) and water (200 g) at the same temperature. Each coin has a specific heat of 390 J/kg•°C and a mass of 8.1 g. Determine the final temperature (T). Assume that the container does not allow heat exchange with surroundings.
A 500 g block of aluminum is heated to 400 ℃ and dropped into water initially at 20 ℃. What is the minimum mass of water (mw) required to cool down the block to 125 ℃?
A 1.20 cm chromium cube (emissivity = 0.28) is used for heating. It is enclosed in a container that is nearly a vacuum, whose walls have a temperature of 283.0 K. Determine the rate of power supply to the cube that will keep it at a temperature of 811 K. Assume conduction by supports is negligible.
Imagine that a student is investigating a spherical blackbody object that has a radius of 1.5 cm and an emission peak wavelength of 4.0 μm. Assuming that the emissivity is 1, calculate what the power radiated will be.
You planned to roast meat on fire. The iron rod that will be used to hold the meat is to be placed horizontally above the fire but unknowingly one end of the rod slipped off the ring that is used to keep the rod horizontal and touched the fire, with the other end still touching the ring. The rod is now in a slanting position. The radius of the rod is 40 mm and the length is 55 cm. The temperature of the fire is 100°C. Determine the time required to transfer 160 J of energy from the fire to the ring. Assume the surrounding temperature to be 30°C.

A typical adult human loses heat to the environment through several mechanisms, including radiation, conduction, and convection. However, sweating is one of the most effective ways to cool down the body on a hot day. Suppose a person is sweating profusely and producing 50 ml of sweat per hour, and the sweat evaporates from the skin's surface, carrying away heat from the body. If the heat of vaporization of water at room temperature is 2.4 × 106 J/kg, calculate the rate at which heat energy (in J/s) is removed from the body due to the evaporation of sweat.
In a chemistry lab, a scientist continuously provides heat to an 800 g sample and records the temperature changes. The final phase change is complete at Q = 200 kJ and the data is presented in the graph as shown. Determine the i) heat of fusion and ii) heat of vaporization for the substance based on the experimental results.

During a demonstration, an instructor plans to use 1320 J of heat to vaporize a sample of isopropyl alcohol, initially at 24°C. What is the maximum mass of isopropyl alcohol that can be successfully vaporized using this amount of heat?
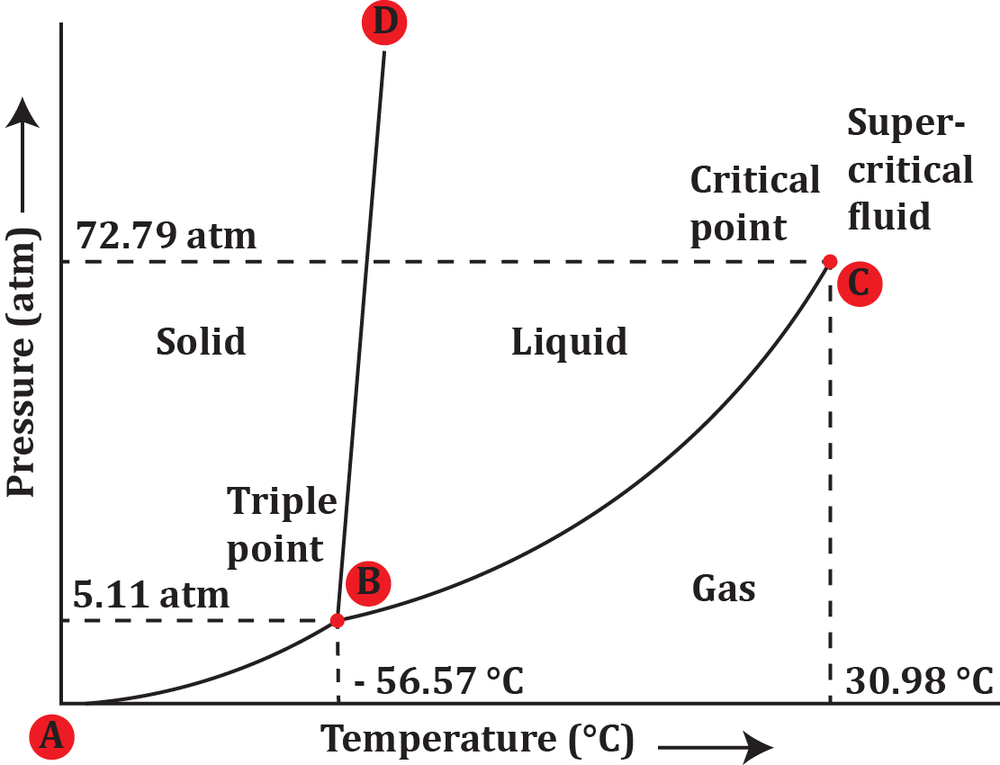
At sea level, in what states can H2O exist? Refer to the phase diagram of H2O.
At a pressure of 5 atm and a temperature of -60°C, determine the phase in which carbon dioxide exists.