Here are the essential concepts you must grasp in order to answer the question correctly.
Manometer Principle
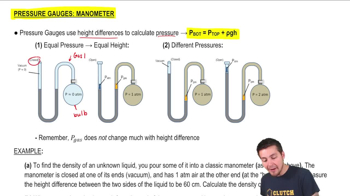
A manometer is a device used to measure pressure by balancing a column of liquid against the pressure to be measured. In an open-tube manometer, the difference in height between two columns of liquid (usually mercury) indicates the pressure difference between the gas in the tank and the atmospheric pressure. The height difference is directly related to the pressure exerted by the gas.
Recommended video:
Pressure Gauges: Manometer
Absolute Pressure
Absolute pressure is the total pressure exerted on a system, measured relative to a perfect vacuum. It is calculated by adding the atmospheric pressure to the gauge pressure (the pressure relative to atmospheric pressure). In this scenario, the absolute pressure in the oxygen tank can be determined by considering the height difference in the mercury column and the atmospheric pressure.
Recommended video:
Pressure and Atmospheric Pressure
Pressure Conversion
Pressure is often measured in various units, such as pascals (Pa), millibars (mbar), and atmospheres (atm). To convert between these units, it is essential to know the relationships: 1 mbar equals 100 Pa. In this problem, converting the atmospheric pressure from mbar to pascals is necessary to find the absolute pressure in the oxygen tank accurately.
Recommended video: