Here are the essential concepts you must grasp in order to answer the question correctly.
Heat Capacity at Constant Volume (Cv)
The heat capacity at constant volume (Cv) is the amount of heat required to raise the temperature of a substance by one degree Celsius (or one Kelvin) while keeping the volume constant. For an ideal gas, Cv depends on the number of degrees of freedom of the gas molecules. Diatomic gases, which have more complex molecular structures than monatomic gases, typically have a higher Cv due to additional rotational and vibrational modes.
Recommended video:
Phase Constant of a Wave Function
Ideal Gas Law
The Ideal Gas Law is a fundamental equation in thermodynamics that relates the pressure, volume, temperature, and number of moles of an ideal gas. It is expressed as PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is temperature in Kelvin. This law helps in understanding how gases behave under various conditions and is essential for calculating changes in state variables.
Recommended video:
Ideal Gases and the Ideal Gas Law
Molar Heat Capacity
Molar heat capacity is the amount of heat required to raise the temperature of one mole of a substance by one degree Celsius (or one Kelvin). For diatomic gases, the molar heat capacity at constant volume (Cv,m) is typically around 5R/2, where R is the ideal gas constant. This concept is crucial for calculating the total heat transfer when the temperature of a gas changes, especially under constant volume conditions.
Recommended video:
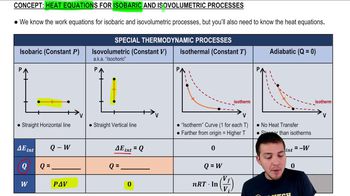
Heat Equations for Isobaric & Isovolumetric Processes
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 6:12m
6:12m