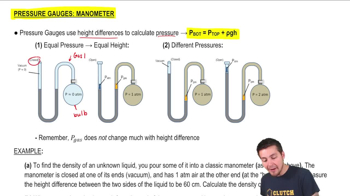
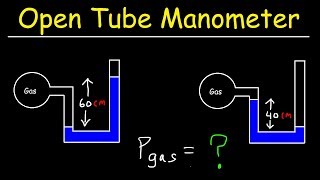
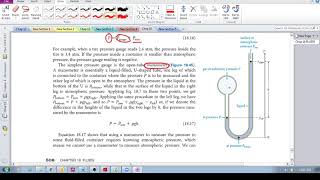
Everybody. Hopefully, you try this practice problem here. So we've got a classic manometer. It's got one of the ends that's open, so we got some kind of atmospheric pressure on the left side, and we've got 2 atmospheres gas on the other. Now we're going to fill this thing with mercury. So remember that mercury here, this is Hg. Remember that the density of mercury is equal to 13,600. You might want to just memorize that. It's really important. We're going to measure the top of the mercury column on the left to be 40 centimeters higher than on the right. So basically, what that means here, remember, is that on the left side, it's going to touch that atmospheric pressure on the left. On the right side, it's going to be the boundary between the two atmosphere gas. You always draw the line straight across over here and this height difference, this h is going to be 0.4. That's going to be 40 centimeters. Now, we're going to calculate the atmospheric pressure in atmospheres that the manometer is exposed to. So what does that mean? We've got 2 atmospheres on the bulb side, and on the left side, it's open, presumably to the air that's on the outside here. Now what you might be thinking is that isn't this atmospheric pressure just 1? And no, it's not. Remember that if they say standard atmospheric pressure, then that's always 1 ATM. But if you're ever asked to calculate the atmospheric pressure, then you cannot assume that it's 1 atmosphere. Right? Remember that pressure changes, like if you were to go up a mountain or something like that. Right? So you could have this instrument somewhere that isn't at standard atmospheric pressure, and this is actually what we want to calculate here. Okay? So whenever you ask for it, you can't just assume it's 1. So how do we do this? Well, remember, we're just going to use our P bottom equation. So you've got:
P bottom = P top + ρ g hSo we got the density of the liquid, we've got the height of the column and g is just a constant. So now what about the top and the bottom pressures? Well, P top remember is always at the top of the column here. So basically, that's what we want to calculate. What is the P top? That's really what we want to find here. The bottom is going to be the pressure of whatever the bulb pressure is. Right? The bottom is basically going to be the same pressure as this, and this is just going to be the same pressure as whatever the gas is. So in other words, this P bottom here is just P gas and the P top here is going to be the P atmospheric pressure. That's really what we really want. So this is going to be a plus. Now we've got 13,600, times 9.8 times 0.4. Remember that P gas here is really just equal to 2 atmospheres. Remember, we can't plug in 2 atmospheres. We have this. We have to convert this to Pascals. Remember that the conversion is that 1 atmosphere is equal to 101,000 Pascals. So if 1 atmosphere is 101,000, then 2 atmospheres is equal to 202,000 pascals. This is going to be ATM. And then what happens is you're going to get:
P atm = 202000 - 53312Now, just moving this over, what you're going to get here is that P atmosphere is equal to 148,688 Pascals. Alright? Just rounding it to the nearest sort of 1,000 here, but that is your answer. But we actually want this we want to calculate this in atmospheres. So now all we have to do is we just have to use this conversion factor again. So we're just going to use:
P atm ÷ 101000to get rid of Pascals, and we're going to get atmospheres and what you should get is 1.47 atmospheres. Alright? Now, it makes sense that we got a number that was less than 2. And the reason for that is this is 2 atmospheres on the right side and this atmospheric pressure on the outside is going to be 1.47. It makes sense that it's less because that means that the 2 atmosphere on the right side is going to push down more in the column than on the left side, and so it's naturally going to go up like this. Okay? So that totally makes sense that we got something that was less than 2. Alright, folks. That's it for this one. Let me know if you have any questions.