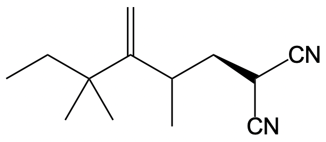
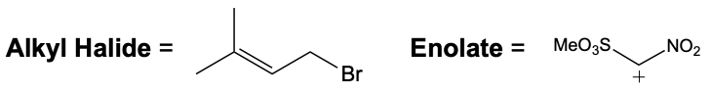
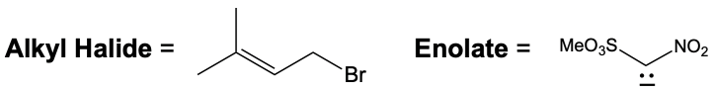
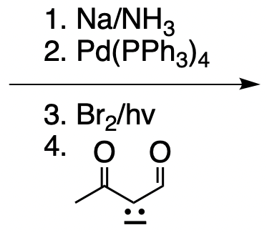
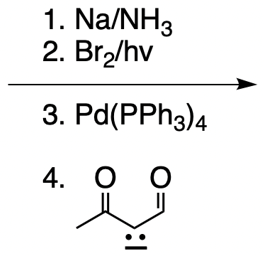
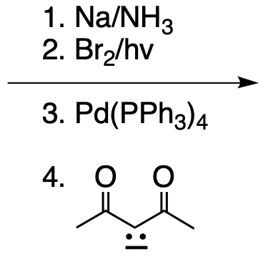
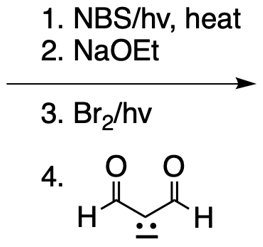
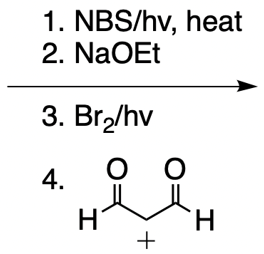
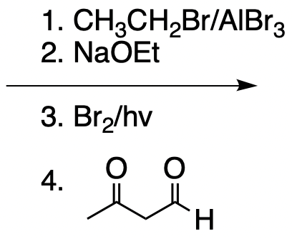
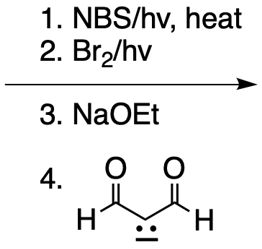
Everyone. In this video, let's take a look at catalytic allylic alkylation. Here, we're going to involve the coupling between an allylic carbon and an enolate. Now if you don't recall what an enolate is, make sure you go back and take a look at our alpha carbon chemistry reactions. We talk about enolates and enols.
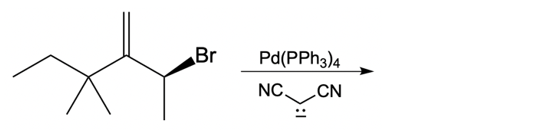
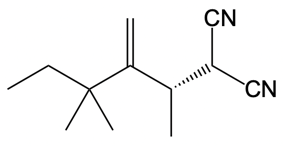
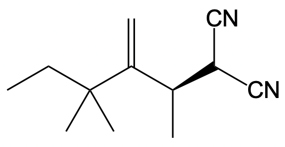
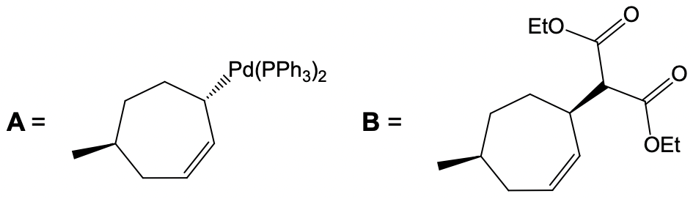
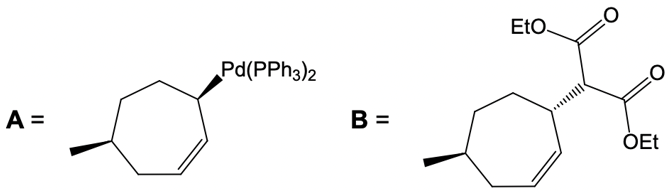
Now here we're talking about an enolate interacting with an allylic carbon. Here we're going to say the reaction itself is highly stereoselective in producing one enantiomer over another. Now we're going to say the reaction occurs by a double SN2 reaction with the retention of the RS configuration of your beginning compound. If we take a look here, we have basically starved this carbon here. This carbon in this case is not only the allylic carbon but it's also chiral in nature.
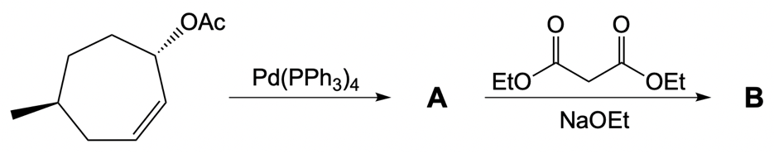
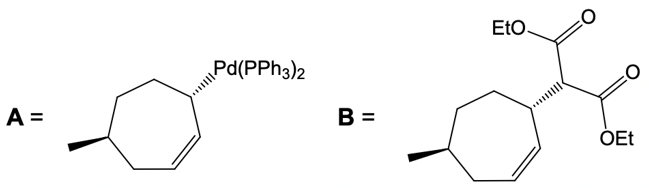
Here our leaving group x has a dashed bond. Here we're going to react it with a Palladium catalyst. This would cause inversion of our configuration, so now the bond is wedged. We have Palladium here. Here, we will be connecting this Palladium to 2 ligands.
Now what we can do here is we can then interact that same chiral position, that allylic carbon with a nucleophile. That nucleophile would do its own SN2 reaction and cause inversion back to our original configuration. So now we'd have here a dash bond like we had originally with our nucleophile attached. Now here, let's talk about this reaction. X represents our leaving group.
This leaving group could be in the form of an ester, or typically a halogen, chlorine, bromine, or iodine. Now recall that many of these catalytic reactions allow esters to be ideal leaving groups. Typically, in a standard SN2 reaction, we're prone to seeing a halogen or maybe a tosylate ion being a good leaving group. In this case, we're also including esters. Now here the palladium catalyst itself could be PD, and we have PPh34, and we could also have palladium(II) chloride.
Now, notice here, this Palladium catalyst has 2 Chlorines attached to it, so these 2 ligands here could be chlorines. But in the case of this one here, Palladium(IV), and here we have triphenylphosphine, although there are going to be 4 of these, only 2 of them will be involved and attached to palladium. So we'd have it like this. That would mean that the other 2 phenyls that we have there would be jettisoned out. We wouldn't need the others, and so just keep that in mind.
Although these two metal catalysts are using Palladium, we have a different number of ligands attached. But at the end of it, we still going to have 2 ligands attached to Palladium once it's done its first SN2 reaction. Now here, the nucleophile as we said before, this is a reaction between an allylic carbon and an enolate. So the nucleophile here would be an enolate group. Alright.
So just keep that in mind when we're looking at the basic setup for this type of reaction.