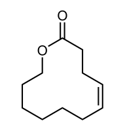
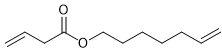
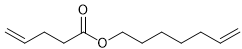
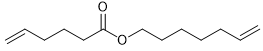
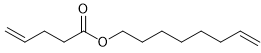
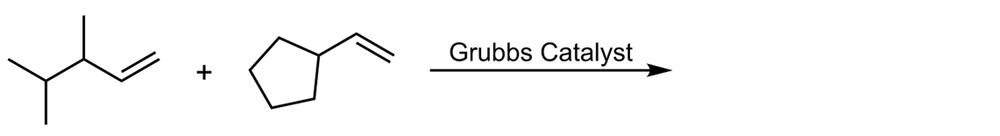
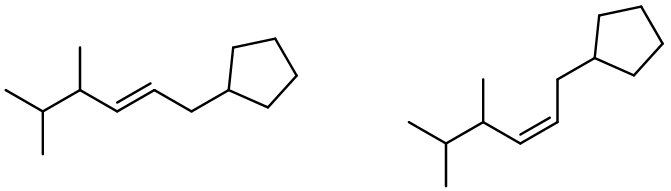
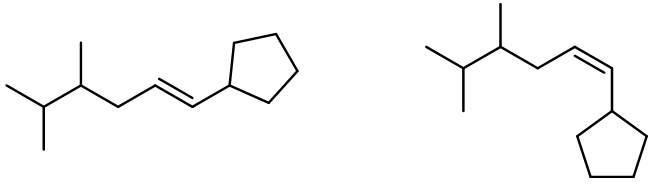
Alkene metathesis, also known as olefin metathesis, is a chemical reaction where two alkenes exchange their carbon atoms at the double bond, resulting in a mixture of E and Z isomers. This process is facilitated by a catalyst known as Grubbs Catalyst, which contains a central ruthenium transition metal bonded to two chlorines and two ligands, with a double bond to a CH group connected to a phenyl group.
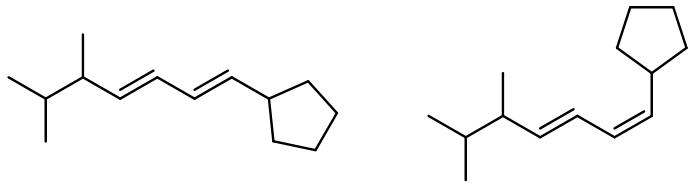
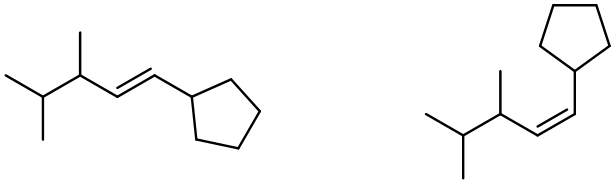
In alkene metathesis, the orientation of the alkenes plays a crucial role. When the alkenes are aligned parallel, the double bonds are effectively "cut," allowing for the formation of new double bonds between the carbon atoms of the two alkenes. This results in various possible alkene products. The E and Z isomers arise from the arrangement of the R groups: if the R groups are on the same side, the product is a Z isomer; if they are on opposite sides, it is an E isomer. Conversely, when the alkenes are oriented oppositely, the resulting alkenes will typically have only one R group each.
Alkene metathesis is characterized as an equilibrium process, with optimal yields achieved when terminal alkenes are utilized. The reaction is driven to completion by the release of ethane gas, which is highly volatile and escapes from the solution, effectively pushing the reaction towards the formation of the desired products.