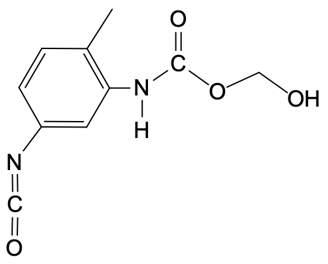
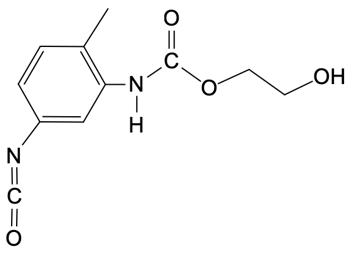
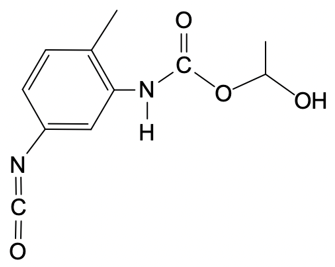
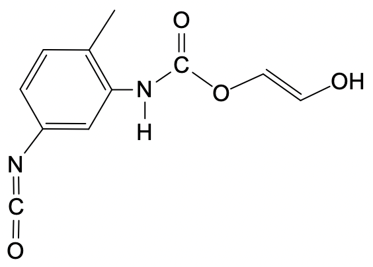
So in this example question, it says, provide the mechanism for the reaction between toluenesocyanate and methylenediol. Alright. So here, I don't specify which version we're dealing with, but the default is 24. So we're talking about the 24 isomer of toluene diisocyanate. Now, the steps are step 1 is nucleophilic attack, and then step 2 is broken down into a and b.
In two way, we're talking about a proton transfer, and in 2b, we're talking about a protonation step. So let's get to it. Step 1, we're going to have our nucleophilic alcohol attacks the carbonyl carbon of our isocyanate. So we're going to take this lone pair, attack right here, which causes this bond to break and go to the oxygen. So what we're going to get here initially is this:
O + O+Oxygen is making 3 bonds so it's positively charged. So there goes our structure. Now step 2a here, this is our deprotonation, which is just another type of proton transfer of the alcohol group by a second diol. So this is going to come, deprotonate this, oxygen holds on to the electrons, so we get this structure. Now this is resonance stabilized because this oxygen here could decide to resonate down and make a pi bond, causing this pi bond to break and go to the nitrogen.
N - N-Now nitrogen is making 2 bonds with 2 lone pairs, so it's negatively charged. And again, remember that this is still here. Right there. And then finally, in 2b, we have protonation of the isocyanate nitrogen by the protonated alcohol to form our urethane at the end. Here we're going to say the urethane represents a repeating monomer that can be elongated as needed.
Okay. So that can be elongated as needed. So if we take a look here, here are the 2 resonance forms. We can say here that this nitrogen with its lone pair comes in deprotonates here, are protonated. And this here would have a hydrogen on it, which is not showing it.
It's positive here, would deprotonate it. And we have protonated the nitrogen to give us at the end our urethane molecule. Alright. So the mechanism itself isn't too bad. It's just 2 simple steps with the second step broken down into 2a and 2b.
So here, we would have our proton transfer step, and then we'd have our protonation step. Right? So just keep this in mind if you're asked to give the mechanism for a urethane formation reaction.