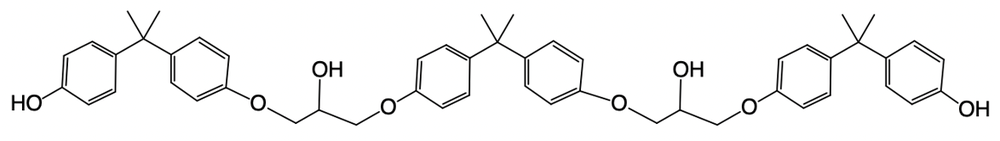
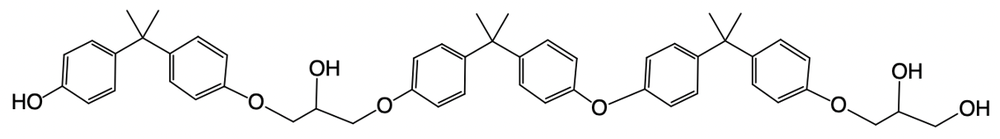
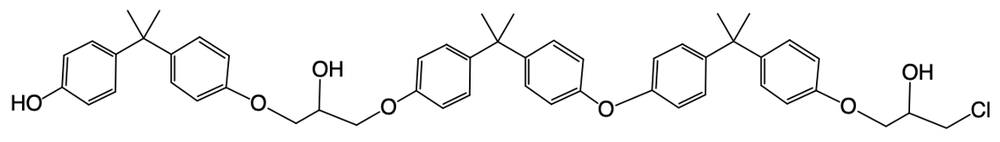
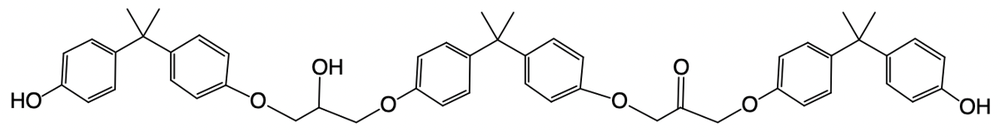
Hey, everyone. So in this example, it says, provide the mechanism for the reaction between 2 moles of BPA and 1 mole of epichlorohydrin within a basic solution. We know that the number of steps is equal to twice the number of BPA plus a protonation step. So twice would mean 4 steps plus a protonation step which is why we have, in this particular question, 5 steps involved. So we have deprotonation and then we have 2a and 2b clumping them together because they're both nucleophilic attacks.
Then we have step 3 which is deprotonation and then nucleophilic attack and then finally, protonation. So let's take a look. In step 1 here, we're talking about our OH- group. We're going to deprotonate it. So we have deprotonation of this BPA molecule, the first hydroxyl group of BPA.
So this comes and deprotonates, oxygen holds on to the electrons, so we have a negatively charged oxygen now. Next, we're gonna say the BPA alkoxide ion attacks the less substituted epoxide carbon via a base catalyzed epoxide ring opening. Remember, this is done under basic conditions. Basic conditions favor base catalyzed epoxide ring opening, which favors an SN2 mechanism. So it's gonna hit this side here causing this to pop open.
So what we're gonna have now is this oxygen is connected to this carbon which is connected to this carbon which is connected to this OH or this O-, but also connected to this Cl. The CH2 and the Cl. Next step, 2b, we're going to say the epoxide ion attacks the epoxide the other epoxide carbon to kick out the chlorine. Alright.
So now it's going to basically attack itself. It's going to do an intramolecular nucleophilic attack. So we come in and we hit here, kick this Cl out. So what do you have here? We have 1, 2.
So 1, 2. And then we'd have this 3, and we recreated an epoxide ring. Now a second mole of BPA is deprotonated for step 3. So, again, we deprotonate here. Oxygen holds on to the electrons, so, again, we have a negative oxygen.
The BPA epoxy alkoxide ion attacks the less substituted epoxide carbon again via a base catalyzed epoxide ring opening. So we're gonna come in, hit this side now causing this to pop open. Alright. So again, it's all about making sure you're tracking the carbons correctly here. Let's make sure we don't mess this up.
So 1, 1, 2, 2, negative oxygen pool. 3, which is this, and we're connecting that to this oxygen, this oxygen here, which is connected to all this stuff. And so we have that now. And then the final step is just our protonation step. So we're gonna have protonation of the conjugate base anion by water forms the epoxy resin.
So here is our negative oxygen. It's gonna come in, grab an H from water. Oxygen holds on to the electrons. So what we get at the end here is our OH group. So this would represent our epoxy resin at the end.
Right? So quite a lengthy mechanism. But, again, a lot of it incorporates things that we know, things that we've learned in previous chapters. We know deprotonation. We know when we're dealing with epoxides in a basic environment that we're gonna do an SN2 mechanism to open that epoxide up.
These steps are essential to create our epoxy resin at the end, which again comes from a combination of our epichlorohydrin as well as our BPA molecule.