Hey guys. So I just want to kick off this topic by describing substitution in the most general terms possible and also by relating it to reactions that we've already learned previously in Organic Chemistry 1. So let's get started. All right. So previously, when we were talking about acids and bases, we talked about how electrons would always move in a very predictable fashion. They would always move from one thing to the other. Okay? And what we said was that basically electrons would always travel from regions of high density to regions of low density. Okay? I've been saying that pretty much all semester, but it holds true again for substitution. Okay? So what that means is that nucleophiles or things with negative charges are going to be attacking electrophiles. All right? So there's actually a lot of different ways that this can happen. Like I've said earlier, a lot of organic chemistry can be broken down just into nucleophiles and electrophiles, but the exact way that they react together is going to be what we actually name as a reaction. Okay?
- 1. A Review of General Chemistry5h 5m
- Summary23m
- Intro to Organic Chemistry5m
- Atomic Structure16m
- Wave Function9m
- Molecular Orbitals17m
- Sigma and Pi Bonds9m
- Octet Rule12m
- Bonding Preferences12m
- Formal Charges6m
- Skeletal Structure14m
- Lewis Structure20m
- Condensed Structural Formula15m
- Degrees of Unsaturation15m
- Constitutional Isomers14m
- Resonance Structures46m
- Hybridization23m
- Molecular Geometry16m
- Electronegativity22m
- 2. Molecular Representations1h 14m
- 3. Acids and Bases2h 46m
- 4. Alkanes and Cycloalkanes4h 19m
- IUPAC Naming29m
- Alkyl Groups13m
- Naming Cycloalkanes10m
- Naming Bicyclic Compounds10m
- Naming Alkyl Halides7m
- Naming Alkenes3m
- Naming Alcohols8m
- Naming Amines15m
- Cis vs Trans21m
- Conformational Isomers13m
- Newman Projections14m
- Drawing Newman Projections16m
- Barrier To Rotation7m
- Ring Strain8m
- Axial vs Equatorial7m
- Cis vs Trans Conformations4m
- Equatorial Preference14m
- Chair Flip9m
- Calculating Energy Difference Between Chair Conformations17m
- A-Values17m
- Decalin7m
- 5. Chirality3h 39m
- Constitutional Isomers vs. Stereoisomers9m
- Chirality12m
- Test 1:Plane of Symmetry7m
- Test 2:Stereocenter Test17m
- R and S Configuration43m
- Enantiomers vs. Diastereomers13m
- Atropisomers9m
- Meso Compound12m
- Test 3:Disubstituted Cycloalkanes13m
- What is the Relationship Between Isomers?16m
- Fischer Projection10m
- R and S of Fischer Projections7m
- Optical Activity5m
- Enantiomeric Excess20m
- Calculations with Enantiomeric Percentages11m
- Non-Carbon Chiral Centers8m
- 6. Thermodynamics and Kinetics1h 22m
- 7. Substitution Reactions1h 48m
- 8. Elimination Reactions2h 30m
- 9. Alkenes and Alkynes2h 9m
- 10. Addition Reactions3h 18m
- Addition Reaction6m
- Markovnikov5m
- Hydrohalogenation6m
- Acid-Catalyzed Hydration17m
- Oxymercuration15m
- Hydroboration26m
- Hydrogenation6m
- Halogenation6m
- Halohydrin12m
- Carbene12m
- Epoxidation8m
- Epoxide Reactions9m
- Dihydroxylation8m
- Ozonolysis7m
- Ozonolysis Full Mechanism24m
- Oxidative Cleavage3m
- Alkyne Oxidative Cleavage6m
- Alkyne Hydrohalogenation3m
- Alkyne Halogenation2m
- Alkyne Hydration6m
- Alkyne Hydroboration2m
- 11. Radical Reactions1h 58m
- 12. Alcohols, Ethers, Epoxides and Thiols2h 42m
- Alcohol Nomenclature4m
- Naming Ethers6m
- Naming Epoxides18m
- Naming Thiols11m
- Alcohol Synthesis7m
- Leaving Group Conversions - Using HX11m
- Leaving Group Conversions - SOCl2 and PBr313m
- Leaving Group Conversions - Sulfonyl Chlorides7m
- Leaving Group Conversions Summary4m
- Williamson Ether Synthesis3m
- Making Ethers - Alkoxymercuration4m
- Making Ethers - Alcohol Condensation4m
- Making Ethers - Acid-Catalyzed Alkoxylation4m
- Making Ethers - Cumulative Practice10m
- Ether Cleavage8m
- Alcohol Protecting Groups3m
- t-Butyl Ether Protecting Groups5m
- Silyl Ether Protecting Groups10m
- Sharpless Epoxidation9m
- Thiol Reactions6m
- Sulfide Oxidation4m
- 13. Alcohols and Carbonyl Compounds2h 17m
- 14. Synthetic Techniques1h 26m
- 15. Analytical Techniques:IR, NMR, Mass Spect7h 3m
- Purpose of Analytical Techniques5m
- Infrared Spectroscopy16m
- Infrared Spectroscopy Table31m
- IR Spect:Drawing Spectra40m
- IR Spect:Extra Practice26m
- NMR Spectroscopy10m
- 1H NMR:Number of Signals26m
- 1H NMR:Q-Test26m
- 1H NMR:E/Z Diastereoisomerism8m
- H NMR Table24m
- 1H NMR:Spin-Splitting (N + 1) Rule22m
- 1H NMR:Spin-Splitting Simple Tree Diagrams11m
- 1H NMR:Spin-Splitting Complex Tree Diagrams12m
- 1H NMR:Spin-Splitting Patterns8m
- NMR Integration18m
- NMR Practice14m
- Carbon NMR4m
- Structure Determination without Mass Spect47m
- Mass Spectrometry12m
- Mass Spect:Fragmentation28m
- Mass Spect:Isotopes27m
- 16. Conjugated Systems6h 13m
- Conjugation Chemistry13m
- Stability of Conjugated Intermediates4m
- Allylic Halogenation12m
- Reactions at the Allylic Position39m
- Conjugated Hydrohalogenation (1,2 vs 1,4 addition)26m
- Diels-Alder Reaction9m
- Diels-Alder Forming Bridged Products11m
- Diels-Alder Retrosynthesis8m
- Molecular Orbital Theory9m
- Drawing Atomic Orbitals6m
- Drawing Molecular Orbitals17m
- HOMO LUMO4m
- Orbital Diagram:3-atoms- Allylic Ions13m
- Orbital Diagram:4-atoms- 1,3-butadiene11m
- Orbital Diagram:5-atoms- Allylic Ions10m
- Orbital Diagram:6-atoms- 1,3,5-hexatriene13m
- Orbital Diagram:Excited States4m
- Pericyclic Reaction10m
- Thermal Cycloaddition Reactions26m
- Photochemical Cycloaddition Reactions26m
- Thermal Electrocyclic Reactions14m
- Photochemical Electrocyclic Reactions10m
- Cumulative Electrocyclic Problems25m
- Sigmatropic Rearrangement17m
- Cope Rearrangement9m
- Claisen Rearrangement15m
- 17. Ultraviolet Spectroscopy51m
- 18. Aromaticity2h 34m
- 19. Reactions of Aromatics: EAS and Beyond5h 1m
- Electrophilic Aromatic Substitution9m
- Benzene Reactions11m
- EAS:Halogenation Mechanism6m
- EAS:Nitration Mechanism9m
- EAS:Friedel-Crafts Alkylation Mechanism6m
- EAS:Friedel-Crafts Acylation Mechanism5m
- EAS:Any Carbocation Mechanism7m
- Electron Withdrawing Groups22m
- EAS:Ortho vs. Para Positions4m
- Acylation of Aniline9m
- Limitations of Friedel-Crafts Alkyation19m
- Advantages of Friedel-Crafts Acylation6m
- Blocking Groups - Sulfonic Acid12m
- EAS:Synergistic and Competitive Groups13m
- Side-Chain Halogenation6m
- Side-Chain Oxidation4m
- Reactions at Benzylic Positions31m
- Birch Reduction10m
- EAS:Sequence Groups4m
- EAS:Retrosynthesis29m
- Diazo Replacement Reactions6m
- Diazo Sequence Groups5m
- Diazo Retrosynthesis13m
- Nucleophilic Aromatic Substitution28m
- Benzyne16m
- 20. Phenols55m
- 21. Aldehydes and Ketones: Nucleophilic Addition4h 56m
- Naming Aldehydes8m
- Naming Ketones7m
- Oxidizing and Reducing Agents9m
- Oxidation of Alcohols28m
- Ozonolysis7m
- DIBAL5m
- Alkyne Hydration9m
- Nucleophilic Addition8m
- Cyanohydrin11m
- Organometallics on Ketones19m
- Overview of Nucleophilic Addition of Solvents13m
- Hydrates6m
- Hemiacetal9m
- Acetal12m
- Acetal Protecting Group16m
- Thioacetal6m
- Imine vs Enamine15m
- Addition of Amine Derivatives5m
- Wolff Kishner Reduction7m
- Baeyer-Villiger Oxidation39m
- Acid Chloride to Ketone7m
- Nitrile to Ketone9m
- Wittig Reaction18m
- Ketone and Aldehyde Synthesis Reactions14m
- 22. Carboxylic Acid Derivatives: NAS2h 51m
- Carboxylic Acid Derivatives7m
- Naming Carboxylic Acids9m
- Diacid Nomenclature6m
- Naming Esters5m
- Naming Nitriles3m
- Acid Chloride Nomenclature5m
- Naming Anhydrides7m
- Naming Amides5m
- Nucleophilic Acyl Substitution18m
- Carboxylic Acid to Acid Chloride6m
- Fischer Esterification5m
- Acid-Catalyzed Ester Hydrolysis4m
- Saponification3m
- Transesterification5m
- Lactones, Lactams and Cyclization Reactions10m
- Carboxylation5m
- Decarboxylation Mechanism14m
- Review of Nitriles46m
- 23. The Chemistry of Thioesters, Phophate Ester and Phosphate Anhydrides1h 10m
- 24. Enolate Chemistry: Reactions at the Alpha-Carbon1h 53m
- Tautomerization9m
- Tautomers of Dicarbonyl Compounds6m
- Enolate4m
- Acid-Catalyzed Alpha-Halogentation4m
- Base-Catalyzed Alpha-Halogentation3m
- Haloform Reaction8m
- Hell-Volhard-Zelinski Reaction3m
- Overview of Alpha-Alkylations and Acylations5m
- Enolate Alkylation and Acylation12m
- Enamine Alkylation and Acylation16m
- Beta-Dicarbonyl Synthesis Pathway7m
- Acetoacetic Ester Synthesis13m
- Malonic Ester Synthesis15m
- 25. Condensation Chemistry2h 9m
- 26. Amines1h 43m
- 27. Heterocycles2h 0m
- Nomenclature of Heterocycles15m
- Acid-Base Properties of Nitrogen Heterocycles10m
- Reactions of Pyrrole, Furan, and Thiophene13m
- Directing Effects in Substituted Pyrroles, Furans, and Thiophenes16m
- Addition Reactions of Furan8m
- EAS Reactions of Pyridine17m
- SNAr Reactions of Pyridine18m
- Side-Chain Reactions of Substituted Pyridines20m
- 28. Carbohydrates5h 53m
- Monosaccharide20m
- Monosaccharides - D and L Isomerism9m
- Monosaccharides - Drawing Fischer Projections18m
- Monosaccharides - Common Structures6m
- Monosaccharides - Forming Cyclic Hemiacetals12m
- Monosaccharides - Cyclization18m
- Monosaccharides - Haworth Projections13m
- Mutarotation11m
- Epimerization9m
- Monosaccharides - Aldose-Ketose Rearrangement8m
- Monosaccharides - Alkylation10m
- Monosaccharides - Acylation7m
- Glycoside6m
- Monosaccharides - N-Glycosides18m
- Monosaccharides - Reduction (Alditols)12m
- Monosaccharides - Weak Oxidation (Aldonic Acid)7m
- Reducing Sugars23m
- Monosaccharides - Strong Oxidation (Aldaric Acid)11m
- Monosaccharides - Oxidative Cleavage27m
- Monosaccharides - Osazones10m
- Monosaccharides - Kiliani-Fischer23m
- Monosaccharides - Wohl Degradation12m
- Monosaccharides - Ruff Degradation12m
- Disaccharide30m
- Polysaccharide11m
- 29. Amino Acids3h 20m
- Proteins and Amino Acids19m
- L and D Amino Acids14m
- Polar Amino Acids14m
- Amino Acid Chart18m
- Acid-Base Properties of Amino Acids33m
- Isoelectric Point14m
- Amino Acid Synthesis: HVZ Method12m
- Synthesis of Amino Acids: Acetamidomalonic Ester Synthesis16m
- Synthesis of Amino Acids: N-Phthalimidomalonic Ester Synthesis13m
- Synthesis of Amino Acids: Strecker Synthesis13m
- Reactions of Amino Acids: Esterification7m
- Reactions of Amino Acids: Acylation3m
- Reactions of Amino Acids: Hydrogenolysis6m
- Reactions of Amino Acids: Ninhydrin Test11m
- 30. Peptides and Proteins2h 42m
- Peptides12m
- Primary Protein Structure4m
- Secondary Protein Structure17m
- Tertiary Protein Structure11m
- Disulfide Bonds17m
- Quaternary Protein Structure10m
- Summary of Protein Structure7m
- Intro to Peptide Sequencing2m
- Peptide Sequencing: Partial Hydrolysis25m
- Peptide Sequencing: Partial Hydrolysis with Cyanogen Bromide7m
- Peptide Sequencing: Edman Degradation28m
- Merrifield Solid-Phase Peptide Synthesis18m
- 32. Lipids 2h 50m
- 34. Nucleic Acids1h 32m
- 35. Transition Metals5h 33m
- Electron Configuration of Elements45m
- Coordination Complexes20m
- Ligands24m
- Electron Counting10m
- The 18 and 16 Electron Rule13m
- Cross-Coupling General Reactions40m
- Heck Reaction40m
- Stille Reaction13m
- Suzuki Reaction25m
- Sonogashira Coupling Reaction17m
- Fukuyama Coupling Reaction15m
- Kumada Coupling Reaction13m
- Negishi Coupling Reaction16m
- Buchwald-Hartwig Amination Reaction19m
- Eglinton Reaction17m
- 36. Synthetic Polymers1h 49m
- Introduction to Polymers6m
- Chain-Growth Polymers10m
- Radical Polymerization15m
- Cationic Polymerization8m
- Anionic Polymerization8m
- Polymer Stereochemistry3m
- Ziegler-Natta Polymerization4m
- Copolymers6m
- Step-Growth Polymers11m
- Step-Growth Polymers: Urethane6m
- Step-Growth Polymers: Polyurethane Mechanism10m
- Step-Growth Polymers: Epoxy Resin8m
- Polymers Structure and Properties8m
Nucleophilic Substitution - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AISubstitution reactions involve a nucleophile attacking an electrophile, resulting in the formation of a new bond and the departure of a leaving group. Unlike Lewis acid-base reactions, substitution requires breaking a bond due to the absence of an empty orbital. The leaving group, often a conjugate base, is crucial for maintaining the octet rule. Understanding the roles of nucleophiles and electrophiles, along with the concept of leaving groups, is essential for mastering organic synthesis and reaction mechanisms.
We can use reactions we've learned in the past (particularly acid-base reactions) to understand substitution. In fact, substitution is just a category of Lewis acid-base reactions!
Remembering general patterns of reactions.
Video transcript
Remember: Nucleophiles always attack electrophiles!
Let's take a stroll through the reactions we've already learned so we can make this connection.
Bronsted-Lowry Reactions:
Nucleophiles and Electrophiles can react in Bronsted-Lowry Reactions.
Video transcript
Let's talk about one of the simplest ways they could react, which would be to react in an acid-base with a Brønsted-Lowry reaction. So when a nucleophile and electrophile react together to exchange a proton, we call that a Brønsted-Lowry reaction. This is an example that we used earlier for acids and bases. You could see here I have a negative charge and I have a neutral substance. If I were to figure out which one is the nucleophile, what would you say that is? Well, the nucleophile was always the thing that was good at donating electrons, so let's say that would be this thing right here. That species is my nucleophile, which means that invariably the other thing needs to be my electrophile. Even if it's difficult for me to see how it's electrophilic, but it must be because the other thing is a better nucleophile. Let's go ahead and say this is my electrophile here.
Now in this reaction, I would have to figure out, okay, now I know my nucleophile and my electrophile, where does that arrow start from? Remember that with mechanisms, we always start from the nucleophile, so I would know that I need to draw an arrow starting from this negative charge. Now the question is where does it go to? To figure out which atom it's going to go to because there's no positive charge directly drawn. If there were a positive charge, then I would just go ahead and attack that. But the electrophile doesn't have a positive, so I'm going to have to use dipoles to figure out what's the most positive atom in this molecule. So I would say I've got a few different bonds. I've got a carbon-sulfur bond. I've also got a hydrogen-sulfur bond. Which direction would those dipoles go? They would both go towards the sulfur. So what that means is that eventually, my sulfur would have a partial negative and both of these would have partial positives. Now notice that I have a positive on a hydrogen. That means that it's the same thing as me saying I have an acidic hydrogen. Why is that acidic? Because it's going to be easily donated because it already has a partial positive charge, so it's looking for something negative that can attack it. So to finish off this arrow, since I have an acidic hydrogen, that's going to be my electrophile right away. I'm going to go ahead and attack the H. You guys could have predicted that's what's going to happen because I just told you we're doing a Brønsted-Lowry reaction.
So I go ahead and grab that H. What's the next question that I ask myself? Well, the next question is always are we done with the mechanism or do we need to keep drawing? Do you think that we're done with the mechanism just the way it's drawn? No, we're actually not. And the reason is that remember that arrow I just drew represents the sharing of two new electrons. So this is two electrons that are now going to be attached to that H. How many electrons does the H want to have? In total, it only wants to have two electrons. So now it would have four electrons if I donated this new lone pair. So that means is that we're going to follow that predictable rule, which is that if I make a bond, I have to break a bond to preserve the octet of the hydrogen. So obviously, the only thing that I can break is this bond to the sulfur, So I would go ahead and dump the electrons onto the sulfur and since this is a Brønsted-Lowry reaction, I would draw equilibrium arrows, and what I would wind up getting is that now I have my O that now has a new single bond, and that new single bond is to an H because it pulled that H off of the acid. Then I also have to draw my conjugate base. Remember that? And my conjugate base would just be the thing that now it doesn't have a hydrogen anymore, so I would draw my ring structure and then I would draw an S and then I would ask myself how many electrons did the S have before? It had eight. It had let me just draw them in. It had a lone pair here and a lone pair there. So this S would still have those blue electrons from before. But now it's going to have one extra lone paired that came from the breaking bond. So I'm going to go ahead and add that lone pair here and then I would use the formal charge rules to figure out what kind of charges these should have. So the oxygen should be neutral because the oxygen wants to have six electrons, and right now it does have six. But the sulfur should have a negative charge because the sulfur wants to have six as well. It's actually in the same column as oxygen, but it has seven electrons. Notice that this is a very predictable Brønsted-Lowry reaction because what I'm doing is I'm reacting a nucleophile-electrophile, and what I'm getting is an exchange of a hydrogen and the exchange of a lone pair. That's easy. This is what we've already done in acids and bases. Is that cool?
In Bronsted-Lowry reactions, a nucleophile attacks an electrophile with an acidic hydrogen, and removes it.
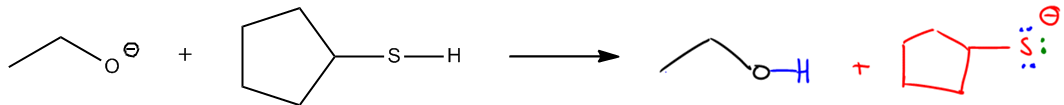
Lewis Reactions:
Nucleophiles and Electrophiles can react in Lewis Acid-Base Reactions.
Video transcript
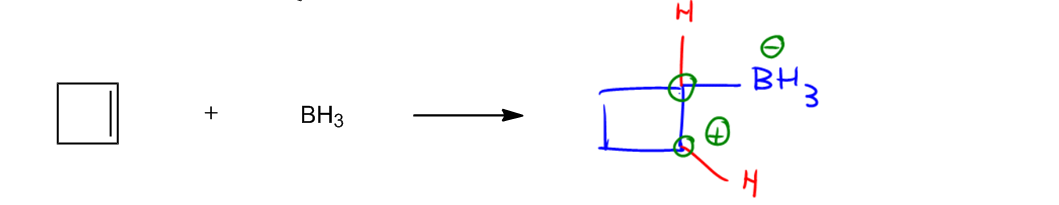
Now what I want to show you guys is how this relates to substitution. Well, remember that we also had the Lewis acid and base definition. That didn't have to do with protons. And what that means is that sometimes you're going to have electrophiles and nucleophiles that want to react together, but there's no acidic hydrogens that they can react with. Does that mean that you give up? No. Okay. You still react and this is an example that I also used when we were talking about acids and bases. We said like in this compound, which one would be the Lewis acid and which one would be the Lewis base? Do you remember? Well, remember that Lewis acid, so I'm just going to write here, is actually the definition of a Lewis acid is that it's an electrophile. Those two words are actually synonymous with each other. Lewis acid means it's a good electron acceptor. Okay? Remember that a Lewis base is synonymous with nucleophile. It means that it's good at giving away electrons. Okay? So in this case, which one would be good at giving away electrons? The double bond. I've mentioned this several times during the span of this course, but I keep saying double bonds are really good sources of electrons. Not electrophiles. Of electrons because they have these 2 free electrons in the pi bond. Okay? So I know that I'm going to start from there. Okay? On top of that, is boron a good electrophile? Actually, yes. Remember that boron and aluminum were 2 special atoms that I keep pointing out that happened to have an incomplete octet or basically they don't have 8 electrons, they only have 6 and they have an empty p orbital. Now if this is the first time that you are hearing me say that, that's okay. That just means that maybe you didn't get to watch the old reviews. Okay? But from now on, this is going to be a very important fact for the rest of Orgo. You need to remember that aluminum and boron are very good at accepting electrons because they just have this empty p orbital that's just waiting to have some electrons in it. So I'm going to go ahead and draw the rest of my mechanism. My electrons would go straight into that orbital. Okay?
So my end products here, when it's a Lewis acid Lewis base, we actually don't use the equilibrium arrows. We use just a forward arrow. The reason is because what we're going to get is a new covalent bond without the exchange of hydrogens. When you have an exchange of hydrogens, you use an equilibrium sign because the hydrogen could go from one place to another and then it could go back. But with Lewis acid, Lewis base, there's no exchange. Okay? So as you can see from my description, I didn't read it, but that's because I wanted to show you guys. When a nucleophile and electrophile react with an empty orbital, that's called a Lewis acid Lewis base. So this is what we were used to doing in the acid-base chapter. When we had an empty orbital, we would just draw this and what I would get now is a square again. Okay? A cyclobutane. Now you guys know how to name that. And I would get now BH3. Okay? Remember that basically every arrow always has to turn into a sigma bond. Alright? Or a single bond. So now I have that and I just have to figure the formal charges. Are there any formal charges here that I have to worry about? And yes, there are because let's look at the double bond for a second. Anytime you break a double bond, what that means is that you are going to be removing electrons for 2 atoms, not just 1. So this top carbon would have had a hydrogen. This bottom carbon would have also had a hydrogen. Why? Because remember that carbon wants to have 4 bonds, so obviously according to bond line, they need 1 hydrogen each. After the reaction, does that change? Absolutely not. They still have 1 hydrogen each there and there. The only difference is that now one of the carbons is happy. Its octet is filled because it has 4 bonds. The other one is not happy because it only has 3. Okay? So what are the formal charges that I'm going to have to put here? There's going to be a positive charge here because that carbon is missing electrons. There's going to be a negative charge here because boron wants to have 3 bonds and now has 4. Easy. Now we're going to leave this right here. Later on in future chapters, we're actually going to continue. Okay? I'm just going to put a question mark because we don't know what that is yet. Okay? But in the addition chapter, once we get there Oops. Addition. What we're going to find is that this is the precursor to a very important reaction. Okay? So but we're not there yet. But I just want to show you guys that this is another example of an acid base, but this is the Lewis definition. Okay? So once again, you're like okay Johnny, I get it. What does this have to do with substitution? Okay? Finally, let's get to it.
In Lewis reactions, a nucleophile attacks an electrophile without an acidic hydrogen, but with an empty orbital, to yield a covalent bond.
Substitution Reactions:
Nucleophiles and Electrophiles can react in Substitution Reactions.
Video transcript
So a substitution reaction is simply a reaction that takes place when you have a nucleophile and an electrophile, like before. The only difference is that it does not have an empty orbital. If it does not have an empty orbital, that presents a problem. What that problem is is that before, when I made the bond for this Lewis acid-Lewis base, did I have to break a bond? No, I didn't because if you have an empty orbital, that means you can accept electrons and you don't need to break a bond after because you're not violating any octets. That boron was ready to accept some electrons. It didn't need to break anything. But now for a substitution reaction, if you don't have an empty orbital, what that means is that if you attack an atom, that atom is going to have too many bonds because there were no empty orbitals. So what that means is that in order to make a bond, I'm going to have to break a bond. And since you have to break a bond, what that means you always have to break a bond in substitution. What that means is that we're going to get a new type of compound called the leaving group. The leaving group is going to simply be the thing that always leaves after the reaction is over. Now we have dealt with leaving groups before. The name that we used to use for the leaving group was just the conjugate base. The conjugate base, if you think about it, it's always the thing that leaves, but that's when you have an acid-base reaction. In these types of reactions, it's not going to be a Brønsted-Lowry acid because we're not going to be exchanging hydrogens but we still are going to have the general principle of something that needs to leave at the end. All right? So I know I've been doing a lot of talking. Let me show you a substitution reaction in action. That rhymed. Wow.
In substitution reactions, a nucleophile attacks an electrophile without an acidic hydrogen, or an empty orbital, so a covalent bond MUST be broken to preserve the octet of the electrophile.
- The bond that is broken to allow this reaction to take place makes a leaving group.
Predict the product
Video transcript
So let's go for it. Nucleophile, electrophile. Which one's the nucleophile? Obviously, my O negative. So let's do that. Nu negative. Which one's my electrophile? Well, it must obviously be the other thing, but which atom is the electrophile? That's the hard part. So what do you guys think? We know that the arrow is going to start where? It has to start at the O. But then where is it going to go? Is it going to go to a carbon, a iodine, a bond? What do you guys think?
In order to listen, if there was a positive charge present on this molecule, that would be easy. But there's no positive charge, so that means my only choice is to use to draw a dipole. So what I have to do here is I have to draw a dipole and the dipole is going to pull in which direction? Well, all these carbon-hydrogen bonds I don't worry about. They're all the same. Okay? They all have very they're all covalent, but I do have a carbon-iodine and that dipole is going to pull towards the iodine. So what that means is that I'm going to have a partial negative here, a partial positive there. Cool so far?
All right. So that means that where is my arrow going to actually attack? It's going to attack the positive because this is a negatively charged nucleophile, which that's kind of the definition of a nucleophile. So now I'm going to go ahead and attack this carbon. Okay? That's already the first common mistake the students make. They don't draw the dipoles and they end up attacking the iodine instead. That doesn't make sense because the iodine has a negative charge already. Why would a negative want to attack a negative?
So now we come to the part with substitution. Okay? Does that carbon have an empty orbital? No. Actually, this carbon already has 4 bonds. Where are those 4? Well, it has 2 to the ring, has 1 to the iodine and it also has one H that we never drew because it was implied. So now I've got a new bond being made to that carbon. How many bonds will that carbon have after that arrow is formed? 5. Does carbon like to be what's called pentavalent or 5 bonds? Absolutely not. That carbon hates its life right now. So what are we going to do to fix this situation because it sucks?
In order to make this bond, I'm going to have to break a bond. In order to break a bond, that means I have to kick out a leaving group. So in this case, I've got 5 bonds. I can choose to break any one of them, but the one that's easiest to break is the one that already has a dipole because the one that already has a dipole is the one that's already pulling electrons away from it, so the bond that I break is the one to the iodine. Afterwards, what I'm going to get is a forward direction arrow not equilibrium. Why? Because this is not the exchange of a proton. It's literally just a nucleophile reacting with an electrophile.
This is like the Lewis acid Lewis base reaction that we did on top. Now all we have to do is we have to draw our products. Well, what would they look like? I would have this ring still, so let's go ahead and draw that ring. I drew it a little bit different. Still a 5-membered ring, though. And what we would notice is that what's attached to that ring? Well, it still has that H, so that H is still there. It doesn't have the I anymore. So let's not draw the I yet. But what it does have is now it's going to have a single bond and that single bond is now going to be attached to my nucleophile, which is O and then an ethyl group. So there we go.
We have a completely new product. In fact, if you were to look at this functional group, this is an ether. Did I start off with an ether? No. I started off with an alkyl halide and now I have an ether. So you can see already we haven't even gotten to the mechanisms, the full mechanisms yet and you can already see how this would be useful. I can make a completely new functional group out of this.
All right? So I've got that thing connected. Is there anything else that I'm missing? The leaving group. Something left to make this reaction possible and that was my I negative. Why is it an I negative? Because now it's going to have one extra lone pair that it didn't have before. Congrats. You guys just drew your first substitution reaction because you were able to use I know I coached you a lot through it, but I'm just letting you guys know we used all the same principles that we used from acid base and now we applied it to substitution and it works. Okay? And the reason is because all the substitution reaction is is it's a Lewis acid Lewis base, electrophile and nucleophile, but when you don't have an empty p orbital. So that means you have to break, make up on and then break up on. And the one that you break is simply the I.
By the way, how could you tell this with substitution? Because notice that everything traded places. My iodine used to be on the ring, now it's in solution. Okay? My negative used to be on the o, and now it's on the I. Okay? So what I'm trying to say here is that everything switched places. The ring used to have an iodine, now the ring has an o. Okay? The O used to have a negative and now the I has a negative. So like everything's perfectly switching places. Okay? And that's the definition of a substitution. Substitution means you're trading things. All right? So a few more facts and then we'll be done with this video really quick. In a typical acid-base reaction, remember that we would use the stability of the conjugate base to determine if it was favored. Okay? What we would say is we would compare pKa's and we'd say which one's stronger, which one's weaker, all that stuff. Well, it turns out that because the conjugate gets a new name in these reactions, we call it a leaving group. We're just going to use the same principle to figure out the reaction rate. We're going to say that the strength or the stability of the leaving group is going to tell us if this is supposed to be a fast reaction or a slow reaction. If it's going to be favored to happen quickly or not favored to happen quickly. All right?
Do you want more practice?
More setsHere’s what students ask on this topic:
What is the difference between SN1 and SN2 reactions?
SN1 (unimolecular nucleophilic substitution) and SN2 (bimolecular nucleophilic substitution) are two types of nucleophilic substitution reactions. In SN1 reactions, the rate-determining step involves the formation of a carbocation intermediate, making the reaction rate dependent on the concentration of the substrate only. SN1 reactions typically occur in tertiary carbons due to carbocation stability. In contrast, SN2 reactions involve a single, concerted step where the nucleophile attacks the substrate simultaneously as the leaving group departs. The rate of SN2 reactions depends on both the substrate and nucleophile concentrations. SN2 reactions are more common in primary carbons due to less steric hindrance.
 Created using AI
Created using AIWhat factors affect the rate of SN2 reactions?
The rate of SN2 reactions is influenced by several factors: 1) Substrate structure: Primary substrates react faster than secondary, while tertiary substrates are usually unreactive due to steric hindrance. 2) Nucleophile strength: Stronger nucleophiles (e.g., OH-, CN-) increase the reaction rate. 3) Leaving group ability: Better leaving groups (e.g., I-, Br-) facilitate faster reactions. 4) Solvent: Polar aprotic solvents (e.g., DMSO, acetone) enhance the rate by stabilizing the nucleophile without solvating it too strongly. 5) Steric hindrance: Bulky groups around the reactive center slow down the reaction.
 Created using AI
Created using AIHow do you determine the leaving group in a nucleophilic substitution reaction?
The leaving group in a nucleophilic substitution reaction is the atom or group that departs with a pair of electrons. Good leaving groups are typically weak bases that can stabilize the negative charge after departure. Common good leaving groups include halides (I-, Br-, Cl-), tosylate (TsO-), and mesylate (MsO-). The ability of a leaving group is often assessed by its conjugate acid's pKa; lower pKa values indicate better leaving groups. For example, HI (pKa ≈ -10) makes I- a better leaving group than HCl (pKa ≈ -7), making Cl- a less effective leaving group.
 Created using AI
Created using AIWhat role do solvents play in nucleophilic substitution reactions?
Solvents significantly influence nucleophilic substitution reactions. In SN1 reactions, polar protic solvents (e.g., water, alcohols) stabilize the carbocation intermediate and the leaving group, facilitating the reaction. In SN2 reactions, polar aprotic solvents (e.g., DMSO, acetone) are preferred as they do not solvate the nucleophile strongly, allowing it to remain reactive. Polar aprotic solvents stabilize the transition state and increase the nucleophile's effectiveness, leading to faster reaction rates. The choice of solvent can thus determine the reaction pathway and rate.
 Created using AI
Created using AIWhy are tertiary carbons more reactive in SN1 reactions but less reactive in SN2 reactions?
Tertiary carbons are more reactive in SN1 reactions because the formation of a stable carbocation intermediate is favored. The stability of the carbocation increases with the number of alkyl groups due to hyperconjugation and inductive effects. However, in SN2 reactions, tertiary carbons are less reactive due to steric hindrance. The bulky groups around the carbon hinder the nucleophile's approach, making it difficult for the nucleophile to attack and displace the leaving group in a single, concerted step.
 Created using AI
Created using AIYour Organic Chemistry tutors
- In contrast to the results of Assessment 13.18, when a secondary haloalkane is treated with sodium ethanethiol...
- Predict the product of the following substitution/addition reactions involving phenoxides. [Because this probl...
- The reaction of an alkyl chloride with potassium iodide is generally carried out in acetone to maximize the am...
- The following functional-group interchange is a useful synthesis of aldehydes.<IMAGE of reaction>(c) Exp...
- What is the product of the reaction of bromoethane with each of the following nucleophiles? a. CH3CH2CH2O− b...
- What is the product of the reaction of bromoethane with each of the following nucleophiles? c. (CH3)3N d. CH3...
- Cardura, a drug used to treat hypertension, is synthesized as shown here. + K2CO3—> A KOH—> B H...
- Strawberry growers have used large quantities of methyl bromide (b.p. 4 °C) to sterilize the soil before plant...
- Which substitution reaction takes place more rapidly? a. CH3CH2Br + H2O or CH3CH2Br + HO−
- Draw all the products of the following reaction:<IMAGE>

