Back when I taught you guys about functional groups, I told you that alkanes actually don't count as a functional group. Okay? Even though they're super abundant, they're everywhere. And the reason that I said that is because it's true. A functional group implies function, that they actually do something, and alkanes really don't react with much at all. Alkanes just come from underground. You dig them up in petroleum. That's what oil is. It's alkanes, and you can't really react with it much. They're super stable. All you can do is blow them up. Okay? You can put them in your car and combust them, but you can't really react with them a whole lot. So, they seem kind of worthless at first glance, but it turns out that there is one thing that they actually can undergo, and that is that they can undergo a radical reaction. Okay? Because radicals are very high energy. So they're going to be able to react with something that's seemingly unreactive, which is alkanes. So I want to show you guys the mechanism by which they do that. So as I just said, alkanes are the backbone of organic molecules, but they're almost completely unreactive. That's why they last for millions of years underground because they don't react with anything. Okay? But there is one thing that they can do in the presence of radicals, and they can add halogens. Okay? So here I have an unreactive hydrocarbon. Like I said, that's from the dinosaurs. It didn't do anything that whole time. Now, I bring it up to the science lab, and I react it with a radical reaction and, lo and behold, I get a halogen on that alkane. Now, what's cool about that is that now I can do a bunch of other types of reactions to it. This is now called a functionalized hydrocarbon. Why? Because now I have a functional group, an alkyl halide. Once you have an alkyl halide, that's the gateway towards organic synthesis because now, guess what? I can do a bunch of stuff so that I can do substitution reactions, elimination reactions, addition reactions, all kinds of stuff because I first added that halogen. Okay? So what I'm going to show you right now is really the first step of all organic synthesis. Okay?
- 1. A Review of General Chemistry5h 5m
- Summary23m
- Intro to Organic Chemistry5m
- Atomic Structure16m
- Wave Function9m
- Molecular Orbitals17m
- Sigma and Pi Bonds9m
- Octet Rule12m
- Bonding Preferences12m
- Formal Charges6m
- Skeletal Structure14m
- Lewis Structure20m
- Condensed Structural Formula15m
- Degrees of Unsaturation15m
- Constitutional Isomers14m
- Resonance Structures46m
- Hybridization23m
- Molecular Geometry16m
- Electronegativity22m
- 2. Molecular Representations1h 14m
- 3. Acids and Bases2h 46m
- 4. Alkanes and Cycloalkanes4h 19m
- IUPAC Naming29m
- Alkyl Groups13m
- Naming Cycloalkanes10m
- Naming Bicyclic Compounds10m
- Naming Alkyl Halides7m
- Naming Alkenes3m
- Naming Alcohols8m
- Naming Amines15m
- Cis vs Trans21m
- Conformational Isomers13m
- Newman Projections14m
- Drawing Newman Projections16m
- Barrier To Rotation7m
- Ring Strain8m
- Axial vs Equatorial7m
- Cis vs Trans Conformations4m
- Equatorial Preference14m
- Chair Flip9m
- Calculating Energy Difference Between Chair Conformations17m
- A-Values17m
- Decalin7m
- 5. Chirality3h 39m
- Constitutional Isomers vs. Stereoisomers9m
- Chirality12m
- Test 1:Plane of Symmetry7m
- Test 2:Stereocenter Test17m
- R and S Configuration43m
- Enantiomers vs. Diastereomers13m
- Atropisomers9m
- Meso Compound12m
- Test 3:Disubstituted Cycloalkanes13m
- What is the Relationship Between Isomers?16m
- Fischer Projection10m
- R and S of Fischer Projections7m
- Optical Activity5m
- Enantiomeric Excess20m
- Calculations with Enantiomeric Percentages11m
- Non-Carbon Chiral Centers8m
- 6. Thermodynamics and Kinetics1h 22m
- 7. Substitution Reactions1h 48m
- 8. Elimination Reactions2h 30m
- 9. Alkenes and Alkynes2h 9m
- 10. Addition Reactions3h 18m
- Addition Reaction6m
- Markovnikov5m
- Hydrohalogenation6m
- Acid-Catalyzed Hydration17m
- Oxymercuration15m
- Hydroboration26m
- Hydrogenation6m
- Halogenation6m
- Halohydrin12m
- Carbene12m
- Epoxidation8m
- Epoxide Reactions9m
- Dihydroxylation8m
- Ozonolysis7m
- Ozonolysis Full Mechanism24m
- Oxidative Cleavage3m
- Alkyne Oxidative Cleavage6m
- Alkyne Hydrohalogenation3m
- Alkyne Halogenation2m
- Alkyne Hydration6m
- Alkyne Hydroboration2m
- 11. Radical Reactions1h 58m
- 12. Alcohols, Ethers, Epoxides and Thiols2h 42m
- Alcohol Nomenclature4m
- Naming Ethers6m
- Naming Epoxides18m
- Naming Thiols11m
- Alcohol Synthesis7m
- Leaving Group Conversions - Using HX11m
- Leaving Group Conversions - SOCl2 and PBr313m
- Leaving Group Conversions - Sulfonyl Chlorides7m
- Leaving Group Conversions Summary4m
- Williamson Ether Synthesis3m
- Making Ethers - Alkoxymercuration4m
- Making Ethers - Alcohol Condensation4m
- Making Ethers - Acid-Catalyzed Alkoxylation4m
- Making Ethers - Cumulative Practice10m
- Ether Cleavage8m
- Alcohol Protecting Groups3m
- t-Butyl Ether Protecting Groups5m
- Silyl Ether Protecting Groups10m
- Sharpless Epoxidation9m
- Thiol Reactions6m
- Sulfide Oxidation4m
- 13. Alcohols and Carbonyl Compounds2h 17m
- 14. Synthetic Techniques1h 26m
- 15. Analytical Techniques:IR, NMR, Mass Spect6h 50m
- Purpose of Analytical Techniques5m
- Infrared Spectroscopy16m
- Infrared Spectroscopy Table31m
- IR Spect:Drawing Spectra40m
- IR Spect:Extra Practice26m
- NMR Spectroscopy10m
- 1H NMR:Number of Signals26m
- 1H NMR:Q-Test26m
- 1H NMR:E/Z Diastereoisomerism8m
- H NMR Table21m
- 1H NMR:Spin-Splitting (N + 1) Rule17m
- 1H NMR:Spin-Splitting Simple Tree Diagrams11m
- 1H NMR:Spin-Splitting Complex Tree Diagrams8m
- 1H NMR:Spin-Splitting Patterns8m
- NMR Integration18m
- NMR Practice14m
- Carbon NMR4m
- Structure Determination without Mass Spect47m
- Mass Spectrometry12m
- Mass Spect:Fragmentation28m
- Mass Spect:Isotopes27m
- 16. Conjugated Systems6h 13m
- Conjugation Chemistry13m
- Stability of Conjugated Intermediates4m
- Allylic Halogenation12m
- Reactions at the Allylic Position39m
- Conjugated Hydrohalogenation (1,2 vs 1,4 addition)26m
- Diels-Alder Reaction9m
- Diels-Alder Forming Bridged Products11m
- Diels-Alder Retrosynthesis8m
- Molecular Orbital Theory9m
- Drawing Atomic Orbitals6m
- Drawing Molecular Orbitals17m
- HOMO LUMO4m
- Orbital Diagram:3-atoms- Allylic Ions13m
- Orbital Diagram:4-atoms- 1,3-butadiene11m
- Orbital Diagram:5-atoms- Allylic Ions10m
- Orbital Diagram:6-atoms- 1,3,5-hexatriene13m
- Orbital Diagram:Excited States4m
- Pericyclic Reaction10m
- Thermal Cycloaddition Reactions26m
- Photochemical Cycloaddition Reactions26m
- Thermal Electrocyclic Reactions14m
- Photochemical Electrocyclic Reactions10m
- Cumulative Electrocyclic Problems25m
- Sigmatropic Rearrangement17m
- Cope Rearrangement9m
- Claisen Rearrangement15m
- 17. Ultraviolet Spectroscopy51m
- 18. Aromaticity2h 31m
- 19. Reactions of Aromatics: EAS and Beyond5h 1m
- Electrophilic Aromatic Substitution9m
- Benzene Reactions11m
- EAS:Halogenation Mechanism6m
- EAS:Nitration Mechanism9m
- EAS:Friedel-Crafts Alkylation Mechanism6m
- EAS:Friedel-Crafts Acylation Mechanism5m
- EAS:Any Carbocation Mechanism7m
- Electron Withdrawing Groups22m
- EAS:Ortho vs. Para Positions4m
- Acylation of Aniline9m
- Limitations of Friedel-Crafts Alkyation19m
- Advantages of Friedel-Crafts Acylation6m
- Blocking Groups - Sulfonic Acid12m
- EAS:Synergistic and Competitive Groups13m
- Side-Chain Halogenation6m
- Side-Chain Oxidation4m
- Reactions at Benzylic Positions31m
- Birch Reduction10m
- EAS:Sequence Groups4m
- EAS:Retrosynthesis29m
- Diazo Replacement Reactions6m
- Diazo Sequence Groups5m
- Diazo Retrosynthesis13m
- Nucleophilic Aromatic Substitution28m
- Benzyne16m
- 20. Phenols55m
- 21. Aldehydes and Ketones: Nucleophilic Addition4h 56m
- Naming Aldehydes8m
- Naming Ketones7m
- Oxidizing and Reducing Agents9m
- Oxidation of Alcohols28m
- Ozonolysis7m
- DIBAL5m
- Alkyne Hydration9m
- Nucleophilic Addition8m
- Cyanohydrin11m
- Organometallics on Ketones19m
- Overview of Nucleophilic Addition of Solvents13m
- Hydrates6m
- Hemiacetal9m
- Acetal12m
- Acetal Protecting Group16m
- Thioacetal6m
- Imine vs Enamine15m
- Addition of Amine Derivatives5m
- Wolff Kishner Reduction7m
- Baeyer-Villiger Oxidation39m
- Acid Chloride to Ketone7m
- Nitrile to Ketone9m
- Wittig Reaction18m
- Ketone and Aldehyde Synthesis Reactions14m
- 22. Carboxylic Acid Derivatives: NAS2h 51m
- Carboxylic Acid Derivatives7m
- Naming Carboxylic Acids9m
- Diacid Nomenclature6m
- Naming Esters5m
- Naming Nitriles3m
- Acid Chloride Nomenclature5m
- Naming Anhydrides7m
- Naming Amides5m
- Nucleophilic Acyl Substitution18m
- Carboxylic Acid to Acid Chloride6m
- Fischer Esterification5m
- Acid-Catalyzed Ester Hydrolysis4m
- Saponification3m
- Transesterification5m
- Lactones, Lactams and Cyclization Reactions10m
- Carboxylation5m
- Decarboxylation Mechanism14m
- Review of Nitriles46m
- 23. The Chemistry of Thioesters, Phophate Ester and Phosphate Anhydrides1h 10m
- 24. Enolate Chemistry: Reactions at the Alpha-Carbon1h 53m
- Tautomerization9m
- Tautomers of Dicarbonyl Compounds6m
- Enolate4m
- Acid-Catalyzed Alpha-Halogentation4m
- Base-Catalyzed Alpha-Halogentation3m
- Haloform Reaction8m
- Hell-Volhard-Zelinski Reaction3m
- Overview of Alpha-Alkylations and Acylations5m
- Enolate Alkylation and Acylation12m
- Enamine Alkylation and Acylation16m
- Beta-Dicarbonyl Synthesis Pathway7m
- Acetoacetic Ester Synthesis13m
- Malonic Ester Synthesis15m
- 25. Condensation Chemistry2h 9m
- 26. Amines1h 43m
- 27. Heterocycles2h 0m
- Nomenclature of Heterocycles15m
- Acid-Base Properties of Nitrogen Heterocycles10m
- Reactions of Pyrrole, Furan, and Thiophene13m
- Directing Effects in Substituted Pyrroles, Furans, and Thiophenes16m
- Addition Reactions of Furan8m
- EAS Reactions of Pyridine17m
- SNAr Reactions of Pyridine18m
- Side-Chain Reactions of Substituted Pyridines20m
- 28. Carbohydrates5h 53m
- Monosaccharide20m
- Monosaccharides - D and L Isomerism9m
- Monosaccharides - Drawing Fischer Projections18m
- Monosaccharides - Common Structures6m
- Monosaccharides - Forming Cyclic Hemiacetals12m
- Monosaccharides - Cyclization18m
- Monosaccharides - Haworth Projections13m
- Mutarotation11m
- Epimerization9m
- Monosaccharides - Aldose-Ketose Rearrangement8m
- Monosaccharides - Alkylation10m
- Monosaccharides - Acylation7m
- Glycoside6m
- Monosaccharides - N-Glycosides18m
- Monosaccharides - Reduction (Alditols)12m
- Monosaccharides - Weak Oxidation (Aldonic Acid)7m
- Reducing Sugars23m
- Monosaccharides - Strong Oxidation (Aldaric Acid)11m
- Monosaccharides - Oxidative Cleavage27m
- Monosaccharides - Osazones10m
- Monosaccharides - Kiliani-Fischer23m
- Monosaccharides - Wohl Degradation12m
- Monosaccharides - Ruff Degradation12m
- Disaccharide30m
- Polysaccharide11m
- 29. Amino Acids3h 20m
- Proteins and Amino Acids19m
- L and D Amino Acids14m
- Polar Amino Acids14m
- Amino Acid Chart18m
- Acid-Base Properties of Amino Acids33m
- Isoelectric Point14m
- Amino Acid Synthesis: HVZ Method12m
- Synthesis of Amino Acids: Acetamidomalonic Ester Synthesis16m
- Synthesis of Amino Acids: N-Phthalimidomalonic Ester Synthesis13m
- Synthesis of Amino Acids: Strecker Synthesis13m
- Reactions of Amino Acids: Esterification7m
- Reactions of Amino Acids: Acylation3m
- Reactions of Amino Acids: Hydrogenolysis6m
- Reactions of Amino Acids: Ninhydrin Test11m
- 30. Peptides and Proteins2h 42m
- Peptides12m
- Primary Protein Structure4m
- Secondary Protein Structure17m
- Tertiary Protein Structure11m
- Disulfide Bonds17m
- Quaternary Protein Structure10m
- Summary of Protein Structure7m
- Intro to Peptide Sequencing2m
- Peptide Sequencing: Partial Hydrolysis25m
- Peptide Sequencing: Partial Hydrolysis with Cyanogen Bromide7m
- Peptide Sequencing: Edman Degradation28m
- Merrifield Solid-Phase Peptide Synthesis18m
- 32. Lipids 2h 50m
- 34. Nucleic Acids1h 32m
- 35. Transition Metals5h 33m
- Electron Configuration of Elements45m
- Coordination Complexes20m
- Ligands24m
- Electron Counting10m
- The 18 and 16 Electron Rule13m
- Cross-Coupling General Reactions40m
- Heck Reaction40m
- Stille Reaction13m
- Suzuki Reaction25m
- Sonogashira Coupling Reaction17m
- Fukuyama Coupling Reaction15m
- Kumada Coupling Reaction13m
- Negishi Coupling Reaction16m
- Buchwald-Hartwig Amination Reaction19m
- Eglinton Reaction17m
Free Radical Halogenation - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AIAlkanes, while stable and largely unreactive, can undergo radical reactions, particularly with halogens, transforming into alkyl halides. This process involves three key steps: initiation, where radicals are generated; propagation, where radicals react with alkanes to form new radicals and alkyl halides; and termination, where radicals combine to form stable products. The radical chain reaction allows for complete conversion of alkanes, making alkyl halides valuable intermediates in organic synthesis, enabling further reactions such as substitution and elimination.
Alkanes are the backbone of organic molecules, yet they are almost completely unreactive.
The only reaction alkanes undergo is radical halogenation, the gateway to the rest of organic synthesis.
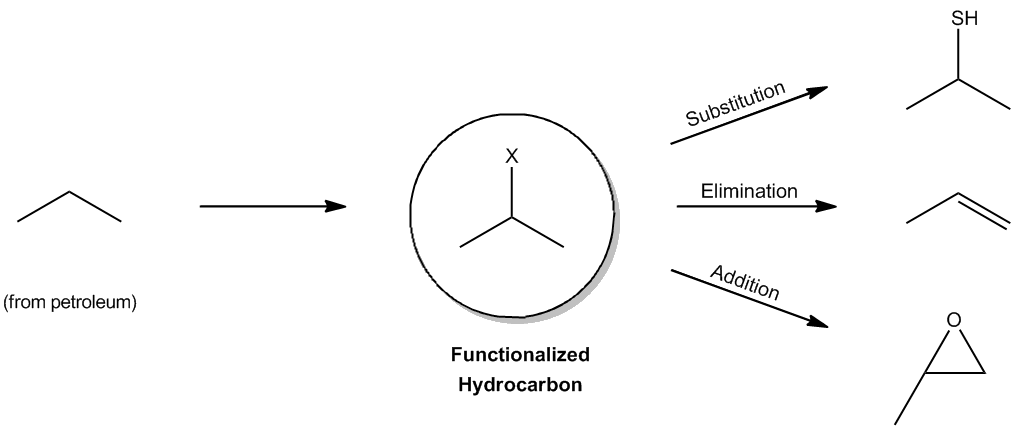
The one reaction that alkanes will actually undergo.
Video transcript
Radical Chain Reaction Mechanism.
Video transcript
So let's just go ahead and talk about it. It turns out that radicals are so high energy that once they react with something, they're going to keep trying to give away that high energy intermediate. Okay? And what winds up happening is that it's like a game of hot potato where no one wants to have the hot potato, so they keep passing it along and it forms what's called a radical chain reaction. Okay? Now the chain reaction, it actually does mean that. It means that once you start it, it actually can't end until it's fully reacted. Until you've fully reacted with all of your alkane. That is useful for us because remember, alkanes aren't that great to begin with, so if we can react them completely, that's a useful reaction as an organic chemist. So let's go ahead and see how this works. Our first step is going to be the initiation step. The initiation step is where I get that first radical because I can't play a game of hot potato without the hot potato itself, so I have to create that first radical. Now, what you notice is that this mechanism is broken down into 3 different steps. And we're actually going to need to write all 3 of these steps. In fact, it's smart that you actually write the words if you do have to draw this mechanism for a test, that you write these three words: Initiation, propagation, and termination. Okay? So let's look at the initiation step. And let's say that we're just using the easiest radical initiator, which is X2. Okay? Let's use X2 over heat. Now, what I taught you guys is that in the initiation step, what we're going to wind up getting is electrons from 2 electrons, 1 on each side, jumping onto each X. So what I'm going to wind up getting is X· + X·. Okay? That's the end of my initiation step. Really all I need for the bare minimum of my initiation step to work, all I need is one radical. In this case, I have 2, so I'm great. Okay? Now that I have that radical in place, that radical is free to react with other molecules. Okay? And it turns out that it reacts really well with alkanes. Now, for the sake of a really simple mechanism, let's just use the simplest alkane possible, which is methane. Methane just being a 1 carbon hydrocarbon. Okay? CH4. So now I've got CH4 and I'm reacting that with X·. Okay? This X· hates itself right now. It's super high energy, super unstable. It's saying, how can I get rid of this hot potato? Can I get rid of it? And then it sees all these electrons in the methane and it's thinking, maybe I can take one of the electrons from one of those carbon-hydrogen bonds. And that's exactly what it does. So it turns out that radicals are going to react with hydrogens and alkanes. And the way we draw these arrows is just so you know, radical reactions are always going to have 3 arrows. So I'm going to draw 1 fishhook into the middle of nowhere. Okay? Then I'm going to draw another fishhook from the CH bond meeting that one. Okay? What that's implying is that now there's going to be a new bond between the H and the X that's going to form from those 2 electrons. So that's looking great, but I still have one electron left over. Notice that the bond between the CH had 2 electrons. So where do you think that last electron goes? It goes onto the C. Okay? It goes onto the carbon backbone. So what that's going to do is it's going to give me a structure that now looks like this: C···HH·. Now notice that I'm drawing the geometry differently because now this would be trigonal planar, right? So you should draw it with like a triangle and that would be + HX. Making sense so far? So notice that the reason this is called a propagation step is that propagation means like I'm reproducing myself. Propagating. And notice that the radical just reproduced itself. Now, it has kind of moved through my medium and now I've got a radical on a new species. Okay? Well, it turns out that your propagation step isn't done yet because you're not done with the propagation step until you fully reproduce yourself. 100%. So what that means is not only do I need to have a radical at the end, I need to have the same exact radical that I started with. So if I start off with an X·, I need to end off with an X·. So what that means is what could I react my C· with to generate that original X· that we had at the beginning? Can you think of anything? It turns out the easiest thing to do is just to react it with another X-X, diatomic halogen. Now, you might be wondering, well, why isn't this already radicals? Because we just did that in the first step. We made it radical. Well, it turns out that not all of the diatomic halogen is going to cleave at the same time. So some of it's going to do the initiation step, but some of it isn't going to be hit by enough light or enough heat to split yet. So what that means is that the one I'm reacting with here hasn't really cleaved yet. Okay? So this is one that's just waiting around for enough energy to finally do that homolytic cleavage. But wait, before the light can even get to it, another radical just did. So instead, we're going to propagate to the X-X. And the way we draw these arrows is once again, 3 arrows. So I'm going to take the radical that always starts it. I'm going to put that one out into the middle of nowhere. Okay? Between the C and then the X. So then I'm going to take one electron from this bond and make it go there. This>
Alkanes will react with diatomic halogens in the presence of heat, light or any other radical initiator.
- Think of the radical as a “hot potato” that the alkanes want to keep passing along!
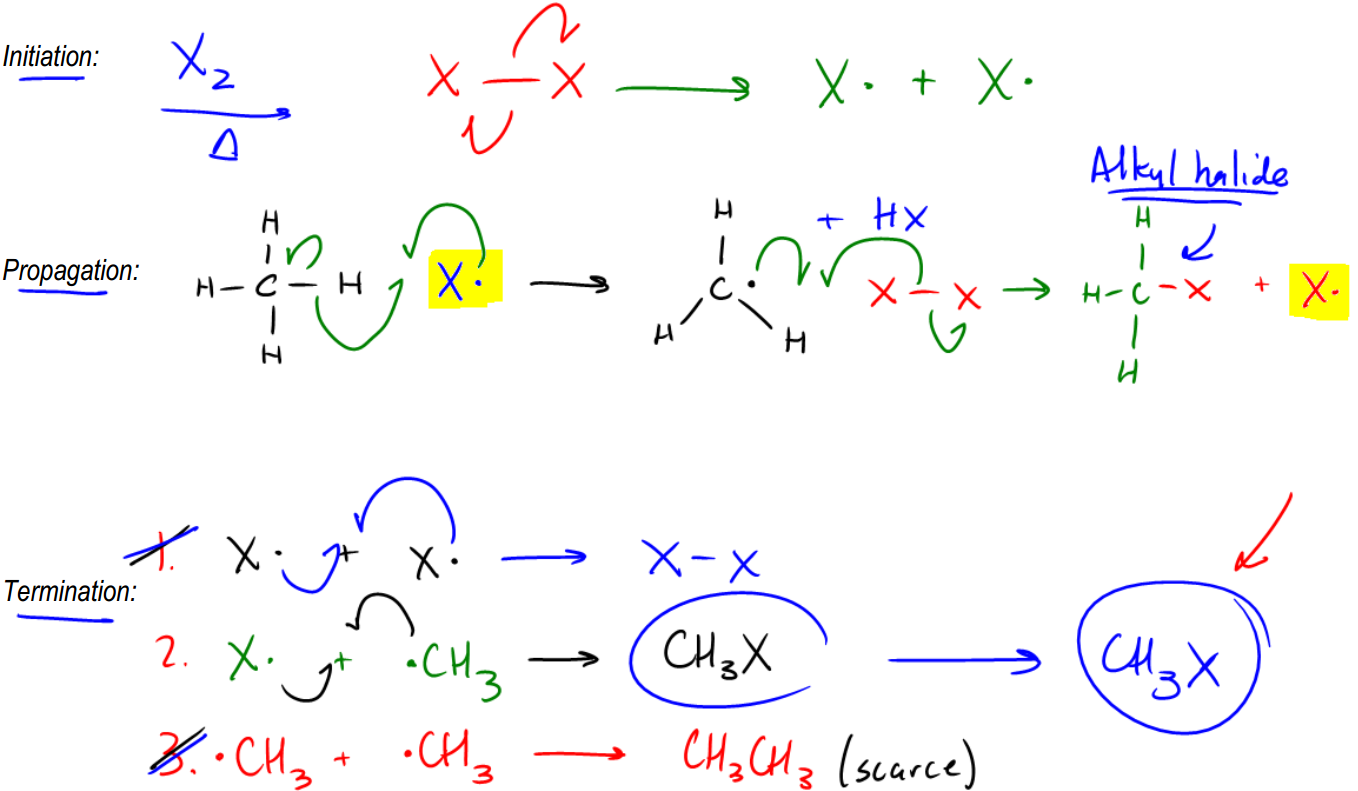
Explaining the following problem.
Video transcript
Now I want you guys to practice the general mechanism for radical halogenation on your own. And I want you guys to notice that this alkane that I'm reacting with has carbons of different stabilities. Okay? I want you guys just to assume that we're going to react with the most stable carbon in this case. So you're going to have to think back to what I talked about with radical stability to figure out which of those hydrogens to pull off in the radical halogenation. I think you guys can get this, though, so I'm just going to let you guys loose on your own, try to draw all 3 steps, and then I'll give you guys the answer. So go for it.
Show the entire chain reaction mechanism.
Video transcript
We know we need to start with the initiation step here. But even before we get to that, I want to ask you guys which hydrogen did you react the radical with, enoyalkane? Basically, there's actually only one choice that made sense, and you should have reacted it with the H right here. The reason is that this H belongs to the only tertiary carbon on this molecule. Now notice that there are no allylic sites here, so I don't worry about the resonance thing. I just worry about which hopefully this will be a learning experience for you. So now let's go ahead and draw the 3 steps. My first step is going to be initiation. I'll just draw it up here to make more room. Okay. So my initiation step is really easy. We're using XX again, so I'm just going to draw like that and like that. I'm going to do this. And what I'm going to wind up getting is 2 X radicals. Cool?
So now let's go into propagation. Now this is the part where it actually matters which hydrogen I used. And I just showed you guys why we're going to use that hydrogen there. I'm going to redraw my alkane and I'm going to draw the hydrogen sticking off this way this time just to make it easier to pull it off. I'm going to react that with x radical. And what that winds up giving me is 3 arrows. 1 here, 1 here, and 1 there. Okay? So what I wind up getting is a radical that looks like this. Okay? That radical plus HX. Okay? Now, what can that radical react with? Well, it in order to fully propagate and reproduce itself, it's going to have to react with another XX. So I'm going to do this, that, and that. And what that's going to give me is it's going to give me an alkyl halide. Notice that this is now a tertiary alkyl halide because I reacted it at a tertiary position, and I'm going to get that final radical. Cool. So now we're just going to end off with the termination step. And with the termination step, we basically had 3 different possibilities. We had X terminating with X. That would be my first product, and that would give me basically that would give me XX. Then we had another possibility, which was now my alkane radical terminating with a halogen radical. And what that would give me is another equivalent of a tertiary alkyl halide. And then, lastly, I had the third possibility, which would be I have basically 2 R groups colliding with each other. Okay? And that would give me a small amount of this kind of random-looking thing, which is going to be, that and then a single bond. And that single bond is attached to basically 2 more methyls and something like that. Okay? So anyway, I know that's ugly. Okay? But the whole point is that I can change the one. In the end of the day, I'm not going to get a whole lot of 1, I'm not going to get a whole lot of 3, but I am going to get a lot of 2. So my final product would be this guy. And then plus, I would get, obviously, a lot of HX as a byproduct. Okay? Because that's going to pretty much be forming all the time. Alright?
Do you want more practice?
More setsHere’s what students ask on this topic:
What is the mechanism of free radical halogenation?
The mechanism of free radical halogenation involves three key steps: initiation, propagation, and termination. In the initiation step, radicals are generated, typically by homolytic cleavage of a diatomic halogen (X2) under heat or light, forming two halogen radicals. During the propagation step, these radicals react with alkanes, forming new radicals and alkyl halides. For example, a halogen radical abstracts a hydrogen atom from methane (CH4), creating a methyl radical (CH3•) and HX. The methyl radical then reacts with another X2 molecule, forming CH3X and another halogen radical. Finally, in the termination step, radicals combine to form stable products, effectively ending the chain reaction.
 Created using AI
Created using AIWhy are alkanes generally unreactive, and how does free radical halogenation change that?
Alkanes are generally unreactive due to their strong C-H and C-C bonds and lack of functional groups, making them stable and resistant to many chemical reactions. However, free radical halogenation changes this by introducing high-energy radicals that can react with the alkanes. The process involves generating radicals that can abstract hydrogen atoms from alkanes, forming alkyl radicals. These alkyl radicals then react with halogen molecules to form alkyl halides, which are much more reactive and can undergo various organic reactions such as substitution and elimination.
 Created using AI
Created using AIWhat are the three steps in the free radical halogenation mechanism?
The three steps in the free radical halogenation mechanism are:
1. Initiation: Radicals are generated, usually by the homolytic cleavage of a diatomic halogen (X2) under heat or light, forming two halogen radicals.
2. Propagation: The halogen radicals react with alkanes to form new radicals and alkyl halides. For example, a halogen radical abstracts a hydrogen atom from an alkane, creating an alkyl radical and HX. The alkyl radical then reacts with another X2 molecule, forming an alkyl halide and another halogen radical.
3. Termination: Radicals combine to form stable products, effectively ending the chain reaction. This can involve the combination of two halogen radicals, an alkyl radical with a halogen radical, or two alkyl radicals.
 Created using AI
Created using AIWhat is the significance of alkyl halides in organic synthesis?
Alkyl halides are significant in organic synthesis because they serve as versatile intermediates for various chemical reactions. Once an alkane is converted into an alkyl halide through free radical halogenation, it can undergo numerous reactions such as nucleophilic substitution, elimination, and addition reactions. These transformations allow for the synthesis of a wide range of organic compounds, making alkyl halides essential building blocks in the field of organic chemistry.
 Created using AI
Created using AIHow does the termination step in free radical halogenation work?
The termination step in free radical halogenation occurs when radicals combine to form stable products, effectively ending the chain reaction. This happens when the concentration of radicals becomes high enough that they are more likely to collide with each other than with unreacted species. Common termination reactions include the combination of two halogen radicals to form X2, an alkyl radical with a halogen radical to form an alkyl halide, or two alkyl radicals to form a larger alkane. These reactions remove radicals from the system, preventing further propagation.
 Created using AI
Created using AIYour Organic Chemistry tutors
- Free-radical chlorination of hexane gives very poor yields of 1-chlorohexane, while cyclohexane can be convert...
- c. How could an industrial plant control the proportions of methane and chlorine to favor production of CCl4? ...
- Peroxides are often added to free-radical reactions as initiators because the oxygen–oxygen bond cleaves homol...
- When exactly 1 mole of methane is mixed with exactly 1 mole of chlorine and light is shone on the mixture, a ...
- Write a mechanism for the light-initiated reaction of cyclohexane with chlorine to give chlorocyclohexane. Lab...
- 3. For each alkane, which monobrominated derivatives could you form in good yield by free-radical bromination?...
- For each alkane, 1. draw all the possible monochlorinated derivatives. c. 2-methylpentane d. 2,2,3,3-tetram...
- The chlorination of pentane gives a mixture of three monochlorinated products. a. Draw their structures.
- Predict the major monohalogenation product(s) of the following reactions. Indicate whether you think the react...
- (••••) When a student attempted a bromination to produce compound A, they generated compound B instead. Ration...
- The radical fluorination of 2-methyl propane resulted in a 14:86 ratio of products. (a) On the basis of this...
- The radical fluorination of 2-methyl propane resulted in a 14:86 ratio of products. (b) From the relative re...
- Show how free-radical halogenation might be used to synthesize the following compounds. In each case, explain...
- Rationalize the fact that reaction A results in an unequal mixture of products, but reaction B yields an equal...
- (••••) Because of the angle strain present in cyclopropanes, they tend to open up in the presence of nearby ra...
- When (1R,3S)-1-tert-butyl-1,3-dimethylcyclopentane is halogenated, one stereoisomer is produced in excess. (a...
- When (1R,3S)-1-tert-butyl-1,3-dimethylcyclopentane is halogenated, one stereoisomer is produced in excess. (b...
- Predict the major products of the following alkane halogenation reactions. [The number of products shown ignor...
- Predict the major products of the following alkane halogenation reactions. [The number of products shown ignor...
- Can you make a 1° bromoalkane like (3-bromopropyl)cyclopentane using alkane halogenation? Why or why not?
- (•) Predict the major product of the following bromination reactions. (b)
- (•) Predict the major product of the following bromination reactions. (c)
- (•••) Suggest a mechanism for the following reactions. (a)
- (•••) For the following reaction, answer questions (a)–(d). (b) Which is the...
- (•••) For the following reaction, answer questions (a)–(d). (d) Given your c...
- Assessments 11.62–11.65 should be answered in order. (•••) A halogenation intended to make compound A formed ...
- Assessments 11.62–11.65 should be answered in order. (•••) A halogenation intended to make compound A formed ...
- In the following reaction, which C―H bond would be most likely to react with a bromine radical?
- Assessments 11.57–11.61 should be answered in order. Each question should be used to help you answer the next....
- Assessments 11.57–11.61 should be answered in order. Each question should be used to help you answer the next....
- Draw the products of the following reactions, including all stereoisomers: d.
- What are the product(s) of each of the following reactions? Disregard stereoisomers. e.
- What are the product(s) of each of the following reactions? Disregard stereoisomers. d.
- What are the product(s) of each of the following reactions? Disregard stereoisomers. c.
- How many alkyl halides are obtained from monochlorination of the alkanes in Problem 4 if stereoisomers are inc...
- How many alkyl halides are obtained from monochlorination of the alkanes in Problem 4 if stereoisomers are inc...
- How many alkyl halides are obtained from monochlorination of the alkanes in Problem 4 if stereoisomers are inc...
- How many alkyl halides are obtained from monochlorination of the alkanes in Problem 4 if stereoisomers are inc...
- How many alkyl halides are obtained from monochlorination of the alkanes in Problem 4 if stereoisomers are inc...
- How many alkyl halides are obtained from monochlorination of the alkanes in Problem 4 if stereoisomers are inc...
- How many alkyl chlorides are obtained from monochlorination of the following alkanes? Disregard stereoisomers....
- How many alkyl chlorides are obtained from monochlorination of the following alkanes? Disregard stereoisomers....
- How many alkyl chlorides are obtained from monochlorination of the following alkanes? Disregard stereoisomers....
- How many alkyl chlorides are obtained from monochlorination of the following alkanes? Disregard stereoisomers....
- How many alkyl chlorides are obtained from monochlorination of the following alkanes? Disregard stereoisomers....
- How many alkyl chlorides are obtained from monochlorination of the following alkanes? Disregard stereoisomers....
- What are the product(s) of each of the following reactions? Disregard stereoisomers. f.
- What are the answers to Problem 29 when the same compounds are treated with Br2 at 125 °C? c.
- What is the major product obtained from treating an excess of each of the following compounds with Cl2 in the ...
- What alkyl halide will be obtained in greatest yield? Ignore stereoisomers. a.
- What alkyl halide will be obtained in greatest yield? Ignore stereoisomers. b.
- a. What hydrocarbon with molecular formula C4H10 forms only two monochlorinated products? Both products are ac...
- b. What hydrocarbon with the same molecular formula as in part a forms three monochlorinated products? One is ...
- Explain why iodine (I2) does not react with ethane, even though I2 is more easily cleaved homolytically than t...
- Write the initiation, propagation, and termination steps for the monochlorination of cyclohexane.
- Provide an arrow-pushing mechanism for the following alkane bromination. [Don't forget to use fishhook arrows ...
- (•••) If a small amount of a moderately nonpolar poisonous compound was added to a pond, why would it be safe...
- Predict the major monohalogenation product(s) of the following reactions. Indicate whether you think the react...
- (••) Predict the product(s) of the following halogenation reactions. Only one equivalent of the halogen is us...
- (••) Predict the product(s) of the following halogenation reactions. Only one equivalent of the halogen is us...
- We have studied the following reactions in previous chapters. For each, (i) indicate which reaction sheets the...
- Provide a mechanism for the chlorination of cyclohexane. Be sure to include initiation, propagation, and three...
- (•••) For the following reaction, answer questions (a)–(d).<IMAGE>(a) Give an arrow-pushing mechanism, i...
- Predict the major products of the following alkane halogenation reactions. [The number of products shown ignor...
- Assessments 11.57–11.61 should be answered in order. Each question should be used to help you answer the next....
- Assessments 11.57–11.61 should be answered in order. Each question should be used to help you answer the next....
- a. What alkane, with molecular formula C5H12, forms only one monochlorinated product when it is heated with Cl...
- Explain why the rate of bromination of methane decreases if HBr is added to the reaction mixture.
- How many alkyl halides are obtained from monochlorination of the alkanes in Problem 4 if stereoisomers are inc...
- c. How many monochlorination products would be obtained if all stereoisomers are included?
- What is the major product obtained from treating an excess of each of the following compounds with Cl2 in the ...
- In the presence of an acid catalyst, acetaldehyde forms a trimer known as paraldehyde. Because it induces slee...
- a. Propose a mechanism for the following reaction:<IMAGE>
- How many alkyl halides are obtained from monochlorination of the alkanes in Problem 4 if stereoisomers are inc...
- a. How many monochlorination products can be obtained from the radical chlorination of methylcyclohexane? Disr...
- Write the steps for formation of tetrachloromethane (CCl4) from the reaction of methane with Cl2 + hv.
- A possible alternative mechanism to that shown in Problem 47 for the monochlorination of methane involves the ...
- Show how you would prepare cyclopentene from each compound.c. cyclopentane (not by dehydrogenation)
- Each of the following proposed mechanisms for the free-radical chlorination of methane is wrong.Explain how th...
- a. Propose a mechanism for the free-radical chlorination of ethane,CH3—CH3 + CL2 hv —> CH3—CH2Cl + HCl
- a. Draw the structure of the transition state for the second propagation step in the chlorination of methane.⋅...
- Using the BDEs in [TABLE 4-2] <IMAGE> (page 167), c. Suggest two reasons why iodine does not react well...
- Alkoxy radicals (R—O•) are generally more stable than alkyl (R⋅)radicals. 1. Write an equation showing an alky...
- Using cyclohexane as one of your starting materials, show how you would synthesize the following compounds. (a...
- When exactly 1 mole of methane is mixed with exactly 1 mole of chlorine and light is shone on the mixture, a ...
- a. Write the propagation steps leading to the formation of dichloromethane (CH2Cl2) from chloromethane.
- Each of the following proposed mechanisms for the free-radical chlorination of methane is wrong. Explain how t...
- a. Propose a mechanism for the free-radical chlorination of ethane, CH3—CH3 + CL2 hv —> CH3—CH2Cl + HCl b. ...
- For each alkane, 1. draw all the possible monochlorinated derivatives.a. Cyclopentane b. Methylcyclopentane
- 2. For each alkane, determine whether free-radical chlorination would be a good way to make any of these monoc...
- b. Explain why free-radical halogenation usually gives mixtures of products.
- c. How many dibrominated products could each of the compounds form if stereoisomers are included?
- a. The following compounds have the same molecular formula as benzene. How many monobrominated products could ...
- Free-radical bromination of the following compound introduces bromine primarily at the benzylic position next ...
- (•••) FROM THE LITERATURE The following transformation was found to occur in areas with large NO₂ emissions. S...
- The human body can excrete drugs and other exogenous molecules by converting them into polar, water-soluble co...
- b. For each reaction, show which stereoisomers are obtained1. NBS/∆/peroxide2. Br2/CH2Cl2
- (••••) One danger associated with storing ether solvents is their tendency to form explosive peroxides when ex...
- a. Which ether is most apt to form a peroxide?
- b. Which ether is least apt to form a peroxide?
- Write an equation for the reaction of vitamin E with an oxidizing radical (RO·) to give ROH and a less reacti...
- A student adds NBS to a solution of 1-methylcyclohexene and irradiates the mixture with a sunlamp until all th...
- For each compound, predict the major product of free-radical bromination. Remember that bromination is highl...
- Using the bond-dissociation energies in Table 5.6, (a) predict whether or not an iodine radical would be se...
- a. What is the major product of the reaction in Problem 7 when the alkane reacts with Cl2 instead of with Br2?...
- Radical addition to alkenes is not effective for the synthesis of iodo- and chloroalkanes. Using your knowledg...
- The benzene ring alters the reactivity of a neighboring group in the benzylic position much as a double bond ...
- Show how the following compounds could be prepared from 2-methylpropane:a. 2-bromo-2-methylpropane

