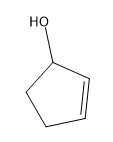
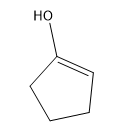
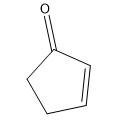
Tautomerization is a phenomenon that naturally happens to all ketones and aldehydes in an aqueous environment. There are base-catalyzed versions and acid-catalyzed versions. We're going to learn both. Essentially, what happens with tautomerization is that whenever you tautomerize a carbonyl, you're going to switch the locations of a pi bond and a hydrogen. What winds up happening is that the hydrogen jumps up to where the pi bond was, and the pi bond jumps down to where the hydrogen was. The relationship between these molecules is called tautomers. They are constitutional isomers of each other because atoms are moving. Please don't call these resonance structures; you can't move atoms in those. Here, you are moving atoms. Like I said, this happens in aqueous environments and happens specifically to ketones and aldehydes. How does this work? The two tautomers have names. The tautomer that normally looks like a carbonyl is called the keto tautomer. It's interesting because it's called the keto tautomer even if it's not a ketone; if it's an aldehyde, you would still call it the keto tautomer. Once you tautomerize, what you're going to make is an alcohol on a double bond, a vinyl alcohol. Vinyl alcohols are special because they can tautomerize. Because you have a vinyl alcohol, this is called the enol tautomer. You've got keto tautomer and enol tautomer. These are in equilibrium with each other in aqueous environments.
Let's look at the acid-catalyzed mechanism of how this happens. In an acid environment, we first protonate, giving us a carbonyl with a positive charge. The conjugate base of the acid can then deprotonate an alpha proton. This deprotonation makes a double bond and kicks the electrons up to the oxygen. This makes the enol tautomer. I have an enol on one side and a keto on the other, and I used acid to make this exchange happen. From now on, this is revolutionary. Anytime that you see a ketone or an aldehyde, you need to be thinking about tautomerization because it's going to happen whether you like it or not.
Let's look at the base-catalyzed version. In a base-catalyzed mechanism, we go straight for the alpha proton right away. We use the oxygen negative to remove the alpha proton, making the double bond and kicking up the electrons. This gives us a negatively charged enol or a very special intermediate called an enolate anion. This makes sense because it's the negatively charged version of enol, so it's called enolate. There's an entire branch of chemistry around enolates, and we're going to spend a lot of time dealing with enolates this semester. They're very special. Because of that, we're going to go through the base-catalyzed mechanism very often because we want to achieve the enolate. To protonate, we would just use the conjugate acid, which would give us the enol. Once again, we have keto and enol. The biggest difference with this one being that with a base-catalyzed mechanism, you pass through this intermediate that's actually very reactive and important. Base catalysis gives you an enolate. That's what tautomerization is. This helps to explain the acidity because the reason that the alpha carbon is so acidic, with a pKa of 20, is that you can form a stable conjugate base or a stable molecule if you remove it because you can always just form the enol. Let's move on to the next video.