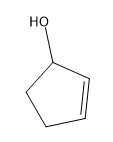
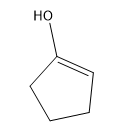
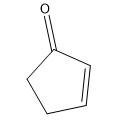
In this video, I'm going to introduce 2 very important new concepts that we need to know for this section. That's the concept of alpha carbons and tautomerization. Previously to this chapter, we've discussed how carbonyl carbons are very reactive because they have a partial positive charge on them. So far, we've always been talking about reactions at the carbonyl carbon. It turns out that carbonyls have another reactive component other than the carbonyl carbon. This is evidenced by pKa values.
I know it's been a long time since your acid and base chapter in Organic Chemistry 1. But does anyone happen to remember what is the pKa of an sp3 hybridized CH bond? Basically an alkane. Do you guys remember what the pKa is of just a typical alkane? I heard someone say it. Great job. It's been forever since we've mentioned that. It's 50. If you said 45, anything above, it's 50. It's something really high, something crazy high. The acidity of a normal alkane is 50. But alpha carbons are uniquely acidic. Alpha carbons don't have a pKa of 50. Guess what their pKa is? 20.
What is responsible for this crazy difference? Just so you guys know, this is on a log scale. That means that it's \(10^{30}\) times more acidic than an alkane. That's an indescribable number. That's a huge, huge number. What could possibly be responsible for this difference? The answer is tautomerization. That's what I'm going to show you guys in the next video.