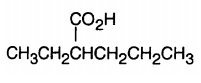Before we can learn to react with carboxylic acids, we need to know how to name them. Let's talk about carboxylic acid nomenclature. There are two really competing and popular ways to name carboxylic acids, and I'm going to teach you both. There's the IUPAC system and the common name system. They're both good in their own ways, and you're going to see them both this semester. Let's start off with the easier one, IUPAC. In the IUPAC system, carboxylic acid is going to be a modifier. You're going to modify your root chain. You take out the 'e' and replace the 'e' with the suffix 'oic acid'. This should make sense. If you think about it, you've heard of benzoic acid before. That's the ending there. Now substituents are located using numbers. The same thing we're used to. If you have a 2-methyl group, you would put 2-methyl. Everything is normal.
The common naming system is a little tricky because it turns out that carboxylic acids are some of the oldest compounds to be studied. They were given names far before the IUPAC convention of 1919. What that means is that sometimes you're going to see carboxylic acid derivatives or carboxylic acids named with the common naming system. Let me give you a case in point. How often have you heard of ethanoic acid? Probably not that often. How often have you heard of acetic acid? You've heard about that since general chemistry. Why do we call it acetic acid? Where does that name come from? It doesn't come from IUPAC. That's a common name. Like acetic acid, we're going to need to learn these common names. The way that we name carboxylic acids with common names is by memorizing the first five common names. The common names have to do with how many carbons are in the chain. A one-carbon carboxylic acid is formic acid. A two-carbon is acetic acid. Three carbons is propionic acid. Four is butyric acid and five is valeric acid. I'm not going to ask you to go more than five. Your professor probably won't either. But then again, it's between you and your professor. I haven't really seen anything more than five in introductory Organic Chemistry. That means that in general, whatever alkane you have, if you're naming it in an IUPAC way, it becomes alkanotic alkanoic acid. That's what we're naming. Just the exact name depends on the identity of the alkane. Also, something I'm kind of jumping around. But I told you, you have to memorize these common names. But it turns out that there's another difference. When you're using common names, you don't locate substituents with numbers. I know. It doesn't make sense. But instead, you're going to use the Greeks. You're going to use Greek symbols. I'm talking about alpha, beta, gamma, delta, epsilon. Again, first five, I don't think you should have to know beyond epsilon. If you're pledging in a sorority or fraternity, you should probably know beyond epsilon. But for right now, that's going to work. We've got these two different systems. You need to know both. Finally, one last thing and then we'll start some practice problems. Whenever you have a negative anion of a carboxylic acid because you know that these things tend to give away their protons. Oftentimes, you'll see a carboxylic acid as an O negative. If you see it as an O negative, you're going to replace 'oic acid' and you're going to replace that with 'ate'. 'Ate' being the suffix for a negatively charged species. Acetic acid turns into acetate when it's a negatively charged species. That should just make sense. What I want you guys to do is look at this first compound. Give me both the IUPAC name and the common name as best as you can do, and then I'll step in and help you guys out.