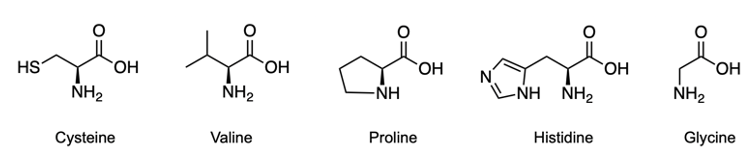
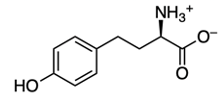
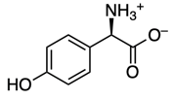
Now we're going to say amino acids are curious compounds that become visibly detectable using ninhydrin. Ninhydrin itself is an aromatic diketone hydrate that reacts with the primary amines of amino acids. When we take a look here, we have our aromatic portion with this benzene, diketone from these two carbonyls, and a hydrate because we have a geminal diol. So, geminal meaning they're on the same carbon. Now, we're going to say this can interact with an amino acid and it creates a dye called Ruhemann's purple.
Now, where's the real-world application? Well, in forensics, it's used to detect fingerprints on surfaces. You might see TV shows or movies where detectives use strips and are able to lift fingerprints off of surfaces. This is ninhydrin interacting with the amino acids in sweat left behind by the person. Yes.
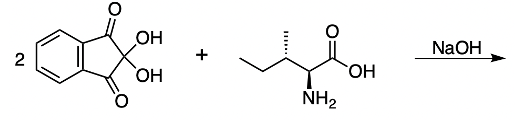
In our sweat, there are bits of amino acids. And the ninhydrin can interact with them leaving behind a fingerprint. Here we can see this purple fingerprint. Now, if we take a look at the reaction itself, we have 2 ninhydrins interacting with 1 amino acid. So it's a 2 to 1 relationship.
We have the use of our base Sodium Hydroxide that creates our ninhydrin here and we're going to say in our ninhydrin, we're going to have a single bond here to nitrogen and a double bond here. Okay. We're going to say this represents our ninhydrin. We're going to say the reaction outcomes of amino acid degradation, one is deamination where the amino group incorporates within the dye. So the nitrogen of the amino group of the amino acid is now here as part of the dye.
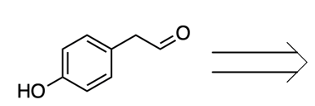
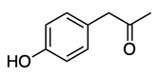
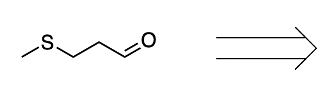
We're going to have decarboxylation. We're going to say here that the carboxyl group here is lost as CO2. And then, finally, we have aldehyde formation. The alpha carbon, so this here gains a double bond O. So, here's the same alpha carbon and it gains a double bond O to create our aldehyde.
So this is the Ninhydrin test. It interacts with the primary amine of the amino acid and it basically breaks down the amino acid into Ruhemann's purple which we would see. And then aldehyde and carbon dioxide as additional byproducts. Right? So, just keep in mind when we're talking about the Ninhydrin test, this is what we mean.
2 moles of a ninhydrin molecule reacting with 1 amino acid to produce our dienaldehyde and carbon dioxide.