All right. So this is a pretty easy example where I'm already starting off with a triple bond, so half the work has already been done for me. All I really need to do is deprotonate it because notice I have a terminal alkyne already. So I don't have to worry about making a triple bond, which is a more advanced part of synthesis. I already have the triple bond. All I need to do is react it with a strong base to get that H off of it, and that's what I'm going to do in my first step. So my first step is NH2− I'm going to grab that H and I'm going to wind up getting an alkynide. Okay? So the alkynide synthesis is the first part. I'm going to go ahead and draw that alkynide down here. And what it's going to look like is just the same thing with a negative. Now I react that alkynide with a leaving group. And in this case, it's a primary leaving group. What do I get when I get a reaction between a strong nucleophile and a primary leaving group? Well, this is the kind of question that we would use my flowchart for because any time you have a leaving group and a strong nucleophile, that's a perfect flowchart question. So you guys might already know the mechanism, but I'm just going to go through this again in case maybe you forgot. By the way, this flowchart that I keep talking about is something that I introduced in the substitution and elimination chapters. Okay? So if you're wondering about this flowchart, just go ahead and look at those videos because we're using that flowchart for a lot of different things. Okay? So, basically and I by the way, I will include and I by the way, I will include a copy of the flowchart in this lesson so that in case you didn't see those, you can still get the copy of the flowchart in this lesson. Okay? But instructions on how to use the flowchart are going to be in those other lessons. Okay? So basically, what we've got is a negatively charged nucleophile, so that would be on the left side of the flowchart. Then 2, what we have is, is it a bulky base? No. So then I would keep going to 3. What kind of leaving group do I have? Primary, so that equals sn2. So this is going to be an sn2 mechanism. Okay? So I would go ahead and draw my alkyl halide, and I would do a backside attack. And what that's going to give me at the end of the second step is now a triple bond with an ethyl group on it. As you can see, I just made my molecule bigger. Now this last step is a reagent that you are supposed to be able to recognize, and this comes from the hydrogenation section. This would be Lindlar's Catalyst. And do you guys remember what Lindlar's catalyst did to triple bonds? It turns them into cis double bonds. Okay? So I don't need to know the mechanism for this. All I need to know is that this is going to become this. Double bond and then ethyl. So notice that now both of my large groups are on the same side of that double bond, making it cis. So congrats guys. You just did your first multistep synthesis. There's going to be a lot more to come in terms of you have to do your own practice, but I'm just trying to show you a really common synthetic pathway. Now, this next one is also very common. Go ahead and try your hardest, and I'll go ahead and give you the answer. Okay? Go for it.
- 1. A Review of General Chemistry5h 5m
- Summary23m
- Intro to Organic Chemistry5m
- Atomic Structure16m
- Wave Function9m
- Molecular Orbitals17m
- Sigma and Pi Bonds9m
- Octet Rule12m
- Bonding Preferences12m
- Formal Charges6m
- Skeletal Structure14m
- Lewis Structure20m
- Condensed Structural Formula15m
- Degrees of Unsaturation15m
- Constitutional Isomers14m
- Resonance Structures46m
- Hybridization23m
- Molecular Geometry16m
- Electronegativity22m
- 2. Molecular Representations1h 14m
- 3. Acids and Bases2h 46m
- 4. Alkanes and Cycloalkanes4h 19m
- IUPAC Naming29m
- Alkyl Groups13m
- Naming Cycloalkanes10m
- Naming Bicyclic Compounds10m
- Naming Alkyl Halides7m
- Naming Alkenes3m
- Naming Alcohols8m
- Naming Amines15m
- Cis vs Trans21m
- Conformational Isomers13m
- Newman Projections14m
- Drawing Newman Projections16m
- Barrier To Rotation7m
- Ring Strain8m
- Axial vs Equatorial7m
- Cis vs Trans Conformations4m
- Equatorial Preference14m
- Chair Flip9m
- Calculating Energy Difference Between Chair Conformations17m
- A-Values17m
- Decalin7m
- 5. Chirality3h 39m
- Constitutional Isomers vs. Stereoisomers9m
- Chirality12m
- Test 1:Plane of Symmetry7m
- Test 2:Stereocenter Test17m
- R and S Configuration43m
- Enantiomers vs. Diastereomers13m
- Atropisomers9m
- Meso Compound12m
- Test 3:Disubstituted Cycloalkanes13m
- What is the Relationship Between Isomers?16m
- Fischer Projection10m
- R and S of Fischer Projections7m
- Optical Activity5m
- Enantiomeric Excess20m
- Calculations with Enantiomeric Percentages11m
- Non-Carbon Chiral Centers8m
- 6. Thermodynamics and Kinetics1h 22m
- 7. Substitution Reactions1h 48m
- 8. Elimination Reactions2h 30m
- 9. Alkenes and Alkynes2h 9m
- 10. Addition Reactions3h 18m
- Addition Reaction6m
- Markovnikov5m
- Hydrohalogenation6m
- Acid-Catalyzed Hydration17m
- Oxymercuration15m
- Hydroboration26m
- Hydrogenation6m
- Halogenation6m
- Halohydrin12m
- Carbene12m
- Epoxidation8m
- Epoxide Reactions9m
- Dihydroxylation8m
- Ozonolysis7m
- Ozonolysis Full Mechanism24m
- Oxidative Cleavage3m
- Alkyne Oxidative Cleavage6m
- Alkyne Hydrohalogenation3m
- Alkyne Halogenation2m
- Alkyne Hydration6m
- Alkyne Hydroboration2m
- 11. Radical Reactions1h 58m
- 12. Alcohols, Ethers, Epoxides and Thiols2h 42m
- Alcohol Nomenclature4m
- Naming Ethers6m
- Naming Epoxides18m
- Naming Thiols11m
- Alcohol Synthesis7m
- Leaving Group Conversions - Using HX11m
- Leaving Group Conversions - SOCl2 and PBr313m
- Leaving Group Conversions - Sulfonyl Chlorides7m
- Leaving Group Conversions Summary4m
- Williamson Ether Synthesis3m
- Making Ethers - Alkoxymercuration4m
- Making Ethers - Alcohol Condensation4m
- Making Ethers - Acid-Catalyzed Alkoxylation4m
- Making Ethers - Cumulative Practice10m
- Ether Cleavage8m
- Alcohol Protecting Groups3m
- t-Butyl Ether Protecting Groups5m
- Silyl Ether Protecting Groups10m
- Sharpless Epoxidation9m
- Thiol Reactions6m
- Sulfide Oxidation4m
- 13. Alcohols and Carbonyl Compounds2h 17m
- 14. Synthetic Techniques1h 26m
- 15. Analytical Techniques:IR, NMR, Mass Spect7h 3m
- Purpose of Analytical Techniques5m
- Infrared Spectroscopy16m
- Infrared Spectroscopy Table31m
- IR Spect:Drawing Spectra40m
- IR Spect:Extra Practice26m
- NMR Spectroscopy10m
- 1H NMR:Number of Signals26m
- 1H NMR:Q-Test26m
- 1H NMR:E/Z Diastereoisomerism8m
- H NMR Table24m
- 1H NMR:Spin-Splitting (N + 1) Rule22m
- 1H NMR:Spin-Splitting Simple Tree Diagrams11m
- 1H NMR:Spin-Splitting Complex Tree Diagrams12m
- 1H NMR:Spin-Splitting Patterns8m
- NMR Integration18m
- NMR Practice14m
- Carbon NMR4m
- Structure Determination without Mass Spect47m
- Mass Spectrometry12m
- Mass Spect:Fragmentation28m
- Mass Spect:Isotopes27m
- 16. Conjugated Systems6h 13m
- Conjugation Chemistry13m
- Stability of Conjugated Intermediates4m
- Allylic Halogenation12m
- Reactions at the Allylic Position39m
- Conjugated Hydrohalogenation (1,2 vs 1,4 addition)26m
- Diels-Alder Reaction9m
- Diels-Alder Forming Bridged Products11m
- Diels-Alder Retrosynthesis8m
- Molecular Orbital Theory9m
- Drawing Atomic Orbitals6m
- Drawing Molecular Orbitals17m
- HOMO LUMO4m
- Orbital Diagram:3-atoms- Allylic Ions13m
- Orbital Diagram:4-atoms- 1,3-butadiene11m
- Orbital Diagram:5-atoms- Allylic Ions10m
- Orbital Diagram:6-atoms- 1,3,5-hexatriene13m
- Orbital Diagram:Excited States4m
- Pericyclic Reaction10m
- Thermal Cycloaddition Reactions26m
- Photochemical Cycloaddition Reactions26m
- Thermal Electrocyclic Reactions14m
- Photochemical Electrocyclic Reactions10m
- Cumulative Electrocyclic Problems25m
- Sigmatropic Rearrangement17m
- Cope Rearrangement9m
- Claisen Rearrangement15m
- 17. Ultraviolet Spectroscopy51m
- 18. Aromaticity2h 34m
- 19. Reactions of Aromatics: EAS and Beyond5h 1m
- Electrophilic Aromatic Substitution9m
- Benzene Reactions11m
- EAS:Halogenation Mechanism6m
- EAS:Nitration Mechanism9m
- EAS:Friedel-Crafts Alkylation Mechanism6m
- EAS:Friedel-Crafts Acylation Mechanism5m
- EAS:Any Carbocation Mechanism7m
- Electron Withdrawing Groups22m
- EAS:Ortho vs. Para Positions4m
- Acylation of Aniline9m
- Limitations of Friedel-Crafts Alkyation19m
- Advantages of Friedel-Crafts Acylation6m
- Blocking Groups - Sulfonic Acid12m
- EAS:Synergistic and Competitive Groups13m
- Side-Chain Halogenation6m
- Side-Chain Oxidation4m
- Reactions at Benzylic Positions31m
- Birch Reduction10m
- EAS:Sequence Groups4m
- EAS:Retrosynthesis29m
- Diazo Replacement Reactions6m
- Diazo Sequence Groups5m
- Diazo Retrosynthesis13m
- Nucleophilic Aromatic Substitution28m
- Benzyne16m
- 20. Phenols55m
- 21. Aldehydes and Ketones: Nucleophilic Addition4h 56m
- Naming Aldehydes8m
- Naming Ketones7m
- Oxidizing and Reducing Agents9m
- Oxidation of Alcohols28m
- Ozonolysis7m
- DIBAL5m
- Alkyne Hydration9m
- Nucleophilic Addition8m
- Cyanohydrin11m
- Organometallics on Ketones19m
- Overview of Nucleophilic Addition of Solvents13m
- Hydrates6m
- Hemiacetal9m
- Acetal12m
- Acetal Protecting Group16m
- Thioacetal6m
- Imine vs Enamine15m
- Addition of Amine Derivatives5m
- Wolff Kishner Reduction7m
- Baeyer-Villiger Oxidation39m
- Acid Chloride to Ketone7m
- Nitrile to Ketone9m
- Wittig Reaction18m
- Ketone and Aldehyde Synthesis Reactions14m
- 22. Carboxylic Acid Derivatives: NAS2h 51m
- Carboxylic Acid Derivatives7m
- Naming Carboxylic Acids9m
- Diacid Nomenclature6m
- Naming Esters5m
- Naming Nitriles3m
- Acid Chloride Nomenclature5m
- Naming Anhydrides7m
- Naming Amides5m
- Nucleophilic Acyl Substitution18m
- Carboxylic Acid to Acid Chloride6m
- Fischer Esterification5m
- Acid-Catalyzed Ester Hydrolysis4m
- Saponification3m
- Transesterification5m
- Lactones, Lactams and Cyclization Reactions10m
- Carboxylation5m
- Decarboxylation Mechanism14m
- Review of Nitriles46m
- 23. The Chemistry of Thioesters, Phophate Ester and Phosphate Anhydrides1h 10m
- 24. Enolate Chemistry: Reactions at the Alpha-Carbon1h 53m
- Tautomerization9m
- Tautomers of Dicarbonyl Compounds6m
- Enolate4m
- Acid-Catalyzed Alpha-Halogentation4m
- Base-Catalyzed Alpha-Halogentation3m
- Haloform Reaction8m
- Hell-Volhard-Zelinski Reaction3m
- Overview of Alpha-Alkylations and Acylations5m
- Enolate Alkylation and Acylation12m
- Enamine Alkylation and Acylation16m
- Beta-Dicarbonyl Synthesis Pathway7m
- Acetoacetic Ester Synthesis13m
- Malonic Ester Synthesis15m
- 25. Condensation Chemistry2h 9m
- 26. Amines1h 43m
- 27. Heterocycles2h 0m
- Nomenclature of Heterocycles15m
- Acid-Base Properties of Nitrogen Heterocycles10m
- Reactions of Pyrrole, Furan, and Thiophene13m
- Directing Effects in Substituted Pyrroles, Furans, and Thiophenes16m
- Addition Reactions of Furan8m
- EAS Reactions of Pyridine17m
- SNAr Reactions of Pyridine18m
- Side-Chain Reactions of Substituted Pyridines20m
- 28. Carbohydrates5h 53m
- Monosaccharide20m
- Monosaccharides - D and L Isomerism9m
- Monosaccharides - Drawing Fischer Projections18m
- Monosaccharides - Common Structures6m
- Monosaccharides - Forming Cyclic Hemiacetals12m
- Monosaccharides - Cyclization18m
- Monosaccharides - Haworth Projections13m
- Mutarotation11m
- Epimerization9m
- Monosaccharides - Aldose-Ketose Rearrangement8m
- Monosaccharides - Alkylation10m
- Monosaccharides - Acylation7m
- Glycoside6m
- Monosaccharides - N-Glycosides18m
- Monosaccharides - Reduction (Alditols)12m
- Monosaccharides - Weak Oxidation (Aldonic Acid)7m
- Reducing Sugars23m
- Monosaccharides - Strong Oxidation (Aldaric Acid)11m
- Monosaccharides - Oxidative Cleavage27m
- Monosaccharides - Osazones10m
- Monosaccharides - Kiliani-Fischer23m
- Monosaccharides - Wohl Degradation12m
- Monosaccharides - Ruff Degradation12m
- Disaccharide30m
- Polysaccharide11m
- 29. Amino Acids3h 20m
- Proteins and Amino Acids19m
- L and D Amino Acids14m
- Polar Amino Acids14m
- Amino Acid Chart18m
- Acid-Base Properties of Amino Acids33m
- Isoelectric Point14m
- Amino Acid Synthesis: HVZ Method12m
- Synthesis of Amino Acids: Acetamidomalonic Ester Synthesis16m
- Synthesis of Amino Acids: N-Phthalimidomalonic Ester Synthesis13m
- Synthesis of Amino Acids: Strecker Synthesis13m
- Reactions of Amino Acids: Esterification7m
- Reactions of Amino Acids: Acylation3m
- Reactions of Amino Acids: Hydrogenolysis6m
- Reactions of Amino Acids: Ninhydrin Test11m
- 30. Peptides and Proteins2h 42m
- Peptides12m
- Primary Protein Structure4m
- Secondary Protein Structure17m
- Tertiary Protein Structure11m
- Disulfide Bonds17m
- Quaternary Protein Structure10m
- Summary of Protein Structure7m
- Intro to Peptide Sequencing2m
- Peptide Sequencing: Partial Hydrolysis25m
- Peptide Sequencing: Partial Hydrolysis with Cyanogen Bromide7m
- Peptide Sequencing: Edman Degradation28m
- Merrifield Solid-Phase Peptide Synthesis18m
- 31. Catalysis in Organic Reactions1h 30m
- 32. Lipids 2h 50m
- 34. Nucleic Acids1h 32m
- 35. Transition Metals5h 33m
- Electron Configuration of Elements45m
- Coordination Complexes20m
- Ligands24m
- Electron Counting10m
- The 18 and 16 Electron Rule13m
- Cross-Coupling General Reactions40m
- Heck Reaction40m
- Stille Reaction13m
- Suzuki Reaction25m
- Sonogashira Coupling Reaction17m
- Fukuyama Coupling Reaction15m
- Kumada Coupling Reaction13m
- Negishi Coupling Reaction16m
- Buchwald-Hartwig Amination Reaction19m
- Eglinton Reaction17m
- 36. Synthetic Polymers1h 49m
- Introduction to Polymers6m
- Chain-Growth Polymers10m
- Radical Polymerization15m
- Cationic Polymerization8m
- Anionic Polymerization8m
- Polymer Stereochemistry3m
- Ziegler-Natta Polymerization4m
- Copolymers6m
- Step-Growth Polymers11m
- Step-Growth Polymers: Urethane6m
- Step-Growth Polymers: Polyurethane Mechanism10m
- Step-Growth Polymers: Epoxy Resin8m
- Polymers Structure and Properties8m
Alkynide Synthesis - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AIIn organic synthesis, starting with a terminal alkyne allows for the formation of an acetylide ion through deprotonation using a strong base like NH2-. This ion can then undergo an SN2 reaction with a primary alkyl halide, resulting in a larger alkyne. The final step involves hydrogenation using Lindlar's catalyst, converting the triple bond into a cis double bond. Understanding these mechanisms is crucial for mastering multi-step synthesis and the application of nucleophiles and leaving groups in organic reactions.
Let's put it all together and provide the expected product.

Predict the major product.
Video transcript
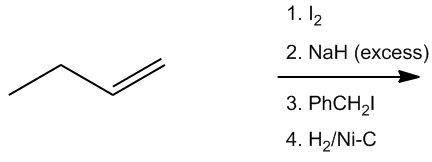
Predict the major product.
Video transcript
So this question starts actually one step further back in the synthetic process where in the previous example, you had a triple bond. But in this example, you aren't even starting with a triple bond, so we're going to have to make the triple bond first and then react it with everything else. So let's go ahead and look at this. I have a double bond with I2. Do you guys know what happens when I react to any type of diatomic halogen with a double bond? It's a reaction that we learned from the addition section of organic chemistry. This would actually be a reaction called halogenation. Now if you don't remember what that is, that's okay, but you do need to know it. What it is, is basically that you would get 2 vicinal halogens together. So, if you guys remember, the mechanism was just kind of like this. I'd have a double bond, I and I, and I would do these three arrows where one arrow goes to the eye, one arrow kicks out the other eye, and one goes back. So what I would wind up getting is a bridge like that. And then the other eye would come in, I negative, would come in and attack. Okay? So at the end of this whole process, what I wind up getting is iodine and iodine. All right? So that's the end of the first step. This is the halogenation step. Okay? So now I have a vicinal dihalide. That's what this is. And do you guys know any way to go from vicinal dihalide to a triple bond? Because that's what I'm trying to do. I'm trying to make triple bonds. And yes, remember that we can take vicinal dihalides or geminal dihalides, and you can react them with excess base to give us triple bonds. Not only that, we can react them with 3 equivalents of base to give us alkynides. So how does that work? Well, I would take this and I would react it with 2 equivalents of base, which in this case is going to be H-. And what that's going to do is it's going to do a double elimination, so I'm going to wind up getti
Let's provide the missing reagents now!
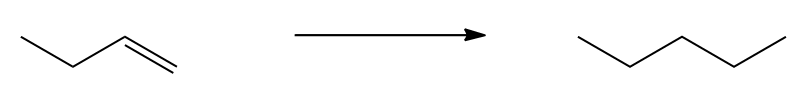
Hints on solving the problem below
Provide the reagents needed

Provide the reagents needed
Video transcript
I hope you guys gave this a fair shot because this is also a very common synthetic pathway. You should know by the end of this lesson how to turn trans into cis and cis into trans. That's a huge part of this chapter. So let's go ahead and get started. Since there's no way to directly turn this into one or the other, I'm going to need to go to the triple bond first. I'm going to need to take it up to a triple bond and then I can hydrogenate it to whatever type of double bond I want. So if I'm making this a triple bond, then I'm going to start off with a diatomic halogen again. Let's try Cl2 this time. What that's going to do is it's going to give me vicinal dihalides. Okay? Then my second step is going to be to make this into a triple bond, and I can do that using one of my strong small bases in excess. Now notice that this is not a terminal triple bond. What this is going to make is actually an internal triple bond where the triple bond is inside of 2 different carbon groups. So could I ever get an alkynide out of this? Remember, an alkynide has that negative charge. No. Because this doesn't even have a hydrogen to remove. There's no hydrogen here. So when I take my base in excess, I'm just going to end at the triple bond. I'm not going to go all the way to the alkynide. Okay? So now I have that triple bond. What could I do to make that into a cis double bond? It's not that hard. All I have to do is use Lindlar's catalyst.
Okay and there were a whole lot of other ways to write it. I'm going to let you guys look that up depending on what your professor wants. But I'm just going to write 'Lind Lars' because that's another acceptable way to write it.
Okay, and it would be 'Lind Lars' and H2. H2 and Lind Lars. And what that's going to give you is that's going to turn that into a cis double bond, and now you've just made the conversion from trans to cis.
And now, you guys should for practice, really before you get to your exam, should know how to go the other way as well, to go from cis to trans. It's not that hard. Okay? So I hope that made sense to you guys. This is just a small taste. At the end of the day, you're going to have to practice, you're going to have to do homework, but I'm just trying to show you, give you hints of stuff that I've seen come up over and over again, and stuff you absolutely can't get to your test without knowing how to do. All right? So let me know if that helped, and let me know if you have questions. Let's move on.
Do you want more practice?
More setsHere’s what students ask on this topic:
What is the role of a strong base in alkynide synthesis?
In alkynide synthesis, a strong base such as NH2- is used to deprotonate a terminal alkyne. This deprotonation removes the hydrogen atom from the terminal alkyne, resulting in the formation of an acetylide ion (alkynide). The acetylide ion is a strong nucleophile, which can then participate in further reactions, such as SN2 reactions with primary alkyl halides, to form larger alkynes. This step is crucial for the synthesis of more complex molecules in organic chemistry.
 Created using AI
Created using AIHow does Lindlar's catalyst affect triple bonds in alkynide synthesis?
Lindlar's catalyst is used in the hydrogenation step of alkynide synthesis to selectively reduce triple bonds to cis double bonds. When a triple bond is treated with Lindlar's catalyst, it adds hydrogen atoms to the alkyne, converting it into a cis-alkene. This means that the two substituents on the carbon atoms of the original triple bond will end up on the same side of the resulting double bond, creating a cis configuration. This selective hydrogenation is important for controlling the stereochemistry of the final product.
 Created using AI
Created using AIWhat is the mechanism of the SN2 reaction in alkynide synthesis?
In alkynide synthesis, the SN2 reaction mechanism involves a nucleophilic attack by the acetylide ion on a primary alkyl halide. The acetylide ion, which is negatively charged, approaches the carbon atom bonded to the leaving group (halide) from the opposite side. This backside attack results in the displacement of the leaving group and the formation of a new carbon-carbon bond. The reaction proceeds in a single step, leading to the inversion of configuration at the carbon center. This mechanism is favored with primary alkyl halides due to minimal steric hindrance.
 Created using AI
Created using AIWhy is it important to understand the flowchart for substitution and elimination reactions in alkynide synthesis?
Understanding the flowchart for substitution and elimination reactions is crucial in alkynide synthesis because it helps determine the appropriate reaction mechanism based on the reactants involved. The flowchart guides you through a series of questions about the nucleophile, base, and leaving group to identify whether the reaction will proceed via SN2, SN1, E2, or E1 mechanisms. This knowledge is essential for predicting the outcome of reactions, optimizing reaction conditions, and designing efficient synthetic pathways in organic chemistry.
 Created using AI
Created using AIWhat are the steps involved in a typical alkynide synthesis reaction?
A typical alkynide synthesis reaction involves three main steps: 1) Deprotonation of a terminal alkyne using a strong base like NH2- to form an acetylide ion. 2) The acetylide ion undergoes an SN2 reaction with a primary alkyl halide, resulting in the formation of a larger alkyne. 3) The final step involves hydrogenation using Lindlar's catalyst, which selectively reduces the triple bond to a cis double bond. These steps are crucial for building more complex molecules and understanding multi-step synthesis in organic chemistry.
 Created using AI
Created using AIYour Organic Chemistry tutors
- c. How much more stable is the most stable staggered conformer than the most stable eclipsed conformer?
- Show how you would synthesize the following compounds, starting with acetylene and any compounds containing ...
- Show how you would synthesize the following compounds, starting with acetylene and any compounds containing ...
- SOLVED PROBLEM 9-1 showed the synthesis of dec-3-yne by adding the hexyl group first, then the ethyl group. Sh...
- (•••) Acetylide alkylation, from Assessment 12.61, fails to give the desired product with 2° haloalkanes. Why?...
- The intended S_N2 displacement of the 1° chloride by acetylide is unsuccessful for the molecule below. Why?
- Show how the following compounds can be synthesized starting with ethyne:b. trans-3-heptene
- Show how each of the following compounds can be synthesized from the given starting materials:a. CH3CH2CH2Br→C...
- How can the following compounds be prepared using ethyne as the starting material?b. <IMAGE>
- How could the following compounds be synthesized from acetylene?c. CH3CH═CH2
- How could the following compounds be synthesized from acetylene?d. <IMAGE>
- How could the following compounds be synthesized from acetylene?e. <IMAGE>
- The fragrance of (Z)-1-phenylhex-2-en-1-ol resembles that of roses, with a delicate citrus edge. Show how you ...
- Show how you would convertd. but-1-yne to cis-hex-3-ene.
- Show how you would synthesize the following compounds from acetylene and any other needed reagents:(a) 6-pheny...
- Muscalure, the sex attractant of the common housefly, is cis-tricos-9-ene. Most syntheses of alkenes give the ...
- For each of the following target molecules, design a multistep synthesis to show how it can be prepared from t...
- Propose structures for intermediates and products (A) through (K)<IMAGE>
- Beginning with acetylene and benzyl bromide and using any other inorganic reagents, propose a synthesis of the...
- Show how the following compounds can be synthesized starting with ethyne: a. cis-2-octene
- Show how you would accomplish the following synthetic transformations. Show all intermediates. (e) < of re...

