Alright, guys. So now let's talk about naming epoxides. It turns out that epoxides are just cyclic ethers. That's basically the definition. And some types of cyclic ethers, remember that an ether is R-O-R, are going to be named as their own functional group due to increased reactivity. Okay? And the specific ones that we usually name as their own functional group are 3-membered ethers. Okay? Because there happens to be a lot of strain in those rings. They're out of their normal bonding preferences or their normal angle preferences. So what that means is that they're very reactive and it's very easy to open them up. And what we call these 3-membered cyclic ethers is there are actually 2 common names for them. We call them epoxides. So go ahead and write that down. Okay? They're also called, in some textbooks, some professors like to use the word oxirane. Okay? Oxirane. These are really synonyms for each other. Okay? An epoxide and an oxirane are the same exact thing. It's just a 3-membered cyclic ether.
- 1. A Review of General Chemistry5h 5m
- Summary23m
- Intro to Organic Chemistry5m
- Atomic Structure16m
- Wave Function9m
- Molecular Orbitals17m
- Sigma and Pi Bonds9m
- Octet Rule12m
- Bonding Preferences12m
- Formal Charges6m
- Skeletal Structure14m
- Lewis Structure20m
- Condensed Structural Formula15m
- Degrees of Unsaturation15m
- Constitutional Isomers14m
- Resonance Structures46m
- Hybridization23m
- Molecular Geometry16m
- Electronegativity22m
- 2. Molecular Representations1h 14m
- 3. Acids and Bases2h 46m
- 4. Alkanes and Cycloalkanes4h 19m
- IUPAC Naming29m
- Alkyl Groups13m
- Naming Cycloalkanes10m
- Naming Bicyclic Compounds10m
- Naming Alkyl Halides7m
- Naming Alkenes3m
- Naming Alcohols8m
- Naming Amines15m
- Cis vs Trans21m
- Conformational Isomers13m
- Newman Projections14m
- Drawing Newman Projections16m
- Barrier To Rotation7m
- Ring Strain8m
- Axial vs Equatorial7m
- Cis vs Trans Conformations4m
- Equatorial Preference14m
- Chair Flip9m
- Calculating Energy Difference Between Chair Conformations17m
- A-Values17m
- Decalin7m
- 5. Chirality3h 39m
- Constitutional Isomers vs. Stereoisomers9m
- Chirality12m
- Test 1:Plane of Symmetry7m
- Test 2:Stereocenter Test17m
- R and S Configuration43m
- Enantiomers vs. Diastereomers13m
- Atropisomers9m
- Meso Compound12m
- Test 3:Disubstituted Cycloalkanes13m
- What is the Relationship Between Isomers?16m
- Fischer Projection10m
- R and S of Fischer Projections7m
- Optical Activity5m
- Enantiomeric Excess20m
- Calculations with Enantiomeric Percentages11m
- Non-Carbon Chiral Centers8m
- 6. Thermodynamics and Kinetics1h 22m
- 7. Substitution Reactions1h 48m
- 8. Elimination Reactions2h 30m
- 9. Alkenes and Alkynes2h 9m
- 10. Addition Reactions3h 18m
- Addition Reaction6m
- Markovnikov5m
- Hydrohalogenation6m
- Acid-Catalyzed Hydration17m
- Oxymercuration15m
- Hydroboration26m
- Hydrogenation6m
- Halogenation6m
- Halohydrin12m
- Carbene12m
- Epoxidation8m
- Epoxide Reactions9m
- Dihydroxylation8m
- Ozonolysis7m
- Ozonolysis Full Mechanism24m
- Oxidative Cleavage3m
- Alkyne Oxidative Cleavage6m
- Alkyne Hydrohalogenation3m
- Alkyne Halogenation2m
- Alkyne Hydration6m
- Alkyne Hydroboration2m
- 11. Radical Reactions1h 58m
- 12. Alcohols, Ethers, Epoxides and Thiols2h 42m
- Alcohol Nomenclature4m
- Naming Ethers6m
- Naming Epoxides18m
- Naming Thiols11m
- Alcohol Synthesis7m
- Leaving Group Conversions - Using HX11m
- Leaving Group Conversions - SOCl2 and PBr313m
- Leaving Group Conversions - Sulfonyl Chlorides7m
- Leaving Group Conversions Summary4m
- Williamson Ether Synthesis3m
- Making Ethers - Alkoxymercuration4m
- Making Ethers - Alcohol Condensation4m
- Making Ethers - Acid-Catalyzed Alkoxylation4m
- Making Ethers - Cumulative Practice10m
- Ether Cleavage8m
- Alcohol Protecting Groups3m
- t-Butyl Ether Protecting Groups5m
- Silyl Ether Protecting Groups10m
- Sharpless Epoxidation9m
- Thiol Reactions6m
- Sulfide Oxidation4m
- 13. Alcohols and Carbonyl Compounds2h 17m
- 14. Synthetic Techniques1h 26m
- 15. Analytical Techniques:IR, NMR, Mass Spect7h 3m
- Purpose of Analytical Techniques5m
- Infrared Spectroscopy16m
- Infrared Spectroscopy Table31m
- IR Spect:Drawing Spectra40m
- IR Spect:Extra Practice26m
- NMR Spectroscopy10m
- 1H NMR:Number of Signals26m
- 1H NMR:Q-Test26m
- 1H NMR:E/Z Diastereoisomerism8m
- H NMR Table24m
- 1H NMR:Spin-Splitting (N + 1) Rule22m
- 1H NMR:Spin-Splitting Simple Tree Diagrams11m
- 1H NMR:Spin-Splitting Complex Tree Diagrams12m
- 1H NMR:Spin-Splitting Patterns8m
- NMR Integration18m
- NMR Practice14m
- Carbon NMR4m
- Structure Determination without Mass Spect47m
- Mass Spectrometry12m
- Mass Spect:Fragmentation28m
- Mass Spect:Isotopes27m
- 16. Conjugated Systems6h 13m
- Conjugation Chemistry13m
- Stability of Conjugated Intermediates4m
- Allylic Halogenation12m
- Reactions at the Allylic Position39m
- Conjugated Hydrohalogenation (1,2 vs 1,4 addition)26m
- Diels-Alder Reaction9m
- Diels-Alder Forming Bridged Products11m
- Diels-Alder Retrosynthesis8m
- Molecular Orbital Theory9m
- Drawing Atomic Orbitals6m
- Drawing Molecular Orbitals17m
- HOMO LUMO4m
- Orbital Diagram:3-atoms- Allylic Ions13m
- Orbital Diagram:4-atoms- 1,3-butadiene11m
- Orbital Diagram:5-atoms- Allylic Ions10m
- Orbital Diagram:6-atoms- 1,3,5-hexatriene13m
- Orbital Diagram:Excited States4m
- Pericyclic Reaction10m
- Thermal Cycloaddition Reactions26m
- Photochemical Cycloaddition Reactions26m
- Thermal Electrocyclic Reactions14m
- Photochemical Electrocyclic Reactions10m
- Cumulative Electrocyclic Problems25m
- Sigmatropic Rearrangement17m
- Cope Rearrangement9m
- Claisen Rearrangement15m
- 17. Ultraviolet Spectroscopy51m
- 18. Aromaticity2h 34m
- 19. Reactions of Aromatics: EAS and Beyond5h 1m
- Electrophilic Aromatic Substitution9m
- Benzene Reactions11m
- EAS:Halogenation Mechanism6m
- EAS:Nitration Mechanism9m
- EAS:Friedel-Crafts Alkylation Mechanism6m
- EAS:Friedel-Crafts Acylation Mechanism5m
- EAS:Any Carbocation Mechanism7m
- Electron Withdrawing Groups22m
- EAS:Ortho vs. Para Positions4m
- Acylation of Aniline9m
- Limitations of Friedel-Crafts Alkyation19m
- Advantages of Friedel-Crafts Acylation6m
- Blocking Groups - Sulfonic Acid12m
- EAS:Synergistic and Competitive Groups13m
- Side-Chain Halogenation6m
- Side-Chain Oxidation4m
- Reactions at Benzylic Positions31m
- Birch Reduction10m
- EAS:Sequence Groups4m
- EAS:Retrosynthesis29m
- Diazo Replacement Reactions6m
- Diazo Sequence Groups5m
- Diazo Retrosynthesis13m
- Nucleophilic Aromatic Substitution28m
- Benzyne16m
- 20. Phenols55m
- 21. Aldehydes and Ketones: Nucleophilic Addition4h 56m
- Naming Aldehydes8m
- Naming Ketones7m
- Oxidizing and Reducing Agents9m
- Oxidation of Alcohols28m
- Ozonolysis7m
- DIBAL5m
- Alkyne Hydration9m
- Nucleophilic Addition8m
- Cyanohydrin11m
- Organometallics on Ketones19m
- Overview of Nucleophilic Addition of Solvents13m
- Hydrates6m
- Hemiacetal9m
- Acetal12m
- Acetal Protecting Group16m
- Thioacetal6m
- Imine vs Enamine15m
- Addition of Amine Derivatives5m
- Wolff Kishner Reduction7m
- Baeyer-Villiger Oxidation39m
- Acid Chloride to Ketone7m
- Nitrile to Ketone9m
- Wittig Reaction18m
- Ketone and Aldehyde Synthesis Reactions14m
- 22. Carboxylic Acid Derivatives: NAS2h 51m
- Carboxylic Acid Derivatives7m
- Naming Carboxylic Acids9m
- Diacid Nomenclature6m
- Naming Esters5m
- Naming Nitriles3m
- Acid Chloride Nomenclature5m
- Naming Anhydrides7m
- Naming Amides5m
- Nucleophilic Acyl Substitution18m
- Carboxylic Acid to Acid Chloride6m
- Fischer Esterification5m
- Acid-Catalyzed Ester Hydrolysis4m
- Saponification3m
- Transesterification5m
- Lactones, Lactams and Cyclization Reactions10m
- Carboxylation5m
- Decarboxylation Mechanism14m
- Review of Nitriles46m
- 23. The Chemistry of Thioesters, Phophate Ester and Phosphate Anhydrides1h 10m
- 24. Enolate Chemistry: Reactions at the Alpha-Carbon1h 53m
- Tautomerization9m
- Tautomers of Dicarbonyl Compounds6m
- Enolate4m
- Acid-Catalyzed Alpha-Halogentation4m
- Base-Catalyzed Alpha-Halogentation3m
- Haloform Reaction8m
- Hell-Volhard-Zelinski Reaction3m
- Overview of Alpha-Alkylations and Acylations5m
- Enolate Alkylation and Acylation12m
- Enamine Alkylation and Acylation16m
- Beta-Dicarbonyl Synthesis Pathway7m
- Acetoacetic Ester Synthesis13m
- Malonic Ester Synthesis15m
- 25. Condensation Chemistry2h 9m
- 26. Amines1h 43m
- 27. Heterocycles2h 0m
- Nomenclature of Heterocycles15m
- Acid-Base Properties of Nitrogen Heterocycles10m
- Reactions of Pyrrole, Furan, and Thiophene13m
- Directing Effects in Substituted Pyrroles, Furans, and Thiophenes16m
- Addition Reactions of Furan8m
- EAS Reactions of Pyridine17m
- SNAr Reactions of Pyridine18m
- Side-Chain Reactions of Substituted Pyridines20m
- 28. Carbohydrates5h 53m
- Monosaccharide20m
- Monosaccharides - D and L Isomerism9m
- Monosaccharides - Drawing Fischer Projections18m
- Monosaccharides - Common Structures6m
- Monosaccharides - Forming Cyclic Hemiacetals12m
- Monosaccharides - Cyclization18m
- Monosaccharides - Haworth Projections13m
- Mutarotation11m
- Epimerization9m
- Monosaccharides - Aldose-Ketose Rearrangement8m
- Monosaccharides - Alkylation10m
- Monosaccharides - Acylation7m
- Glycoside6m
- Monosaccharides - N-Glycosides18m
- Monosaccharides - Reduction (Alditols)12m
- Monosaccharides - Weak Oxidation (Aldonic Acid)7m
- Reducing Sugars23m
- Monosaccharides - Strong Oxidation (Aldaric Acid)11m
- Monosaccharides - Oxidative Cleavage27m
- Monosaccharides - Osazones10m
- Monosaccharides - Kiliani-Fischer23m
- Monosaccharides - Wohl Degradation12m
- Monosaccharides - Ruff Degradation12m
- Disaccharide30m
- Polysaccharide11m
- 29. Amino Acids3h 20m
- Proteins and Amino Acids19m
- L and D Amino Acids14m
- Polar Amino Acids14m
- Amino Acid Chart18m
- Acid-Base Properties of Amino Acids33m
- Isoelectric Point14m
- Amino Acid Synthesis: HVZ Method12m
- Synthesis of Amino Acids: Acetamidomalonic Ester Synthesis16m
- Synthesis of Amino Acids: N-Phthalimidomalonic Ester Synthesis13m
- Synthesis of Amino Acids: Strecker Synthesis13m
- Reactions of Amino Acids: Esterification7m
- Reactions of Amino Acids: Acylation3m
- Reactions of Amino Acids: Hydrogenolysis6m
- Reactions of Amino Acids: Ninhydrin Test11m
- 30. Peptides and Proteins2h 42m
- Peptides12m
- Primary Protein Structure4m
- Secondary Protein Structure17m
- Tertiary Protein Structure11m
- Disulfide Bonds17m
- Quaternary Protein Structure10m
- Summary of Protein Structure7m
- Intro to Peptide Sequencing2m
- Peptide Sequencing: Partial Hydrolysis25m
- Peptide Sequencing: Partial Hydrolysis with Cyanogen Bromide7m
- Peptide Sequencing: Edman Degradation28m
- Merrifield Solid-Phase Peptide Synthesis18m
- 32. Lipids 2h 50m
- 34. Nucleic Acids1h 32m
- 35. Transition Metals5h 33m
- Electron Configuration of Elements45m
- Coordination Complexes20m
- Ligands24m
- Electron Counting10m
- The 18 and 16 Electron Rule13m
- Cross-Coupling General Reactions40m
- Heck Reaction40m
- Stille Reaction13m
- Suzuki Reaction25m
- Sonogashira Coupling Reaction17m
- Fukuyama Coupling Reaction15m
- Kumada Coupling Reaction13m
- Negishi Coupling Reaction16m
- Buchwald-Hartwig Amination Reaction19m
- Eglinton Reaction17m
Naming Epoxides - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AIEpoxides, also known as oxeranes, are three-membered cyclic ethers characterized by significant ring strain, making them highly reactive. Naming conventions include the cycloalkane method, where the ring is named based on its shape, and the epoxy convention, which treats the epoxide as a substituent on the longest carbon chain. Additionally, one can name them as alkenes followed by "oxide," reflecting their formation from double bonds. Understanding these naming systems is crucial for identifying and working with epoxides in organic chemistry.
There are 3 distinct ways to name cyclic ethers. But before we get into them, let’s first specifically define what an epoxide is.
Defining what an epoxide (oxirane) is.
Video transcript
Cycloalkane Convention
Name the ring as a cycloalkane, adding the prefix oxa- and location if necessary.
How to name cyclic ethers using the cycloalkane convention.
Video transcript
Cool so far, right? Now, the challenge becomes how do we name these guys because sometimes, first of all, they're not always three-membered rings. And second of all, there are a lot of substituents. So it turns out that there are 3 different common ways to name epoxides and I'm going to go over all of them right now. Let's start off with what we call the cycloalkane convention. In this type of naming system, what we do is we name the entire ring as if it was an alkane first. Okay? So as you can see here, I have a 6-membered ring. But how many of those atoms are actually carbons? Only 5 of them are. I've got 1, 2, 3, 4, 5. Now you might be wondering why I started the one there. I didn't need to. I'm just using that. I mean maybe I did, but I'm just using that as an example right now just to count carbons. Okay? So I have 5 carbons, but what I'm telling you is that we should actually name it as a cycloalkane, not by the number of carbons. So what that means is that usually when we're naming an alkane, we would say there are 5 carbons, so this would be cyclopentane. But it's not. We're going to call this actually cyclohexane because we go by the shape. What we're worried about here is the shape of the molecule, not how many carbons it has in it. So this would be a cyclohexane first of all as our root. Now the difference is if we have oxygens inside of a ring, which is by definition a cyclic ether, right? Then we're going to add the prefix oxa. And what oxa is going to tell us is that there is one member of this ring that is an oxygen. Okay? So if I call it oxacyclohexane, what I'm saying is that I have a 6-membered ring where one of the atoms is an oxygen, not a carbon. Okay? And then obviously location if necessary. So let's go ahead and just talk about this for a second. The root is going to be the oxacyclohexane. I have that written here. Now we just have to talk about locations. How do we know where to put those guys? Well, it turns out that the oxygen is always going to get your one spot. So when I put the one here, that didn't really count. That wasn't true numbering. The way that I should really number it is starting from the oxygen because that's the highest priority atom inside the ring. And then obviously, I should number to give the lowest overall number or to go to the next highest priority, Okay? So this would be 3-methyl-one oxacyclohexane. Cool so far? Just so you guys know, this also applies to rings that have more than 1 oxygen. If I had 2 oxygens, that would be what was called a dioxa. Okay? Just putting that out there, you could use prefixes as well. Okay? So now let's go ahead and talk about another naming system. This is going to be commonly used for non-three-membered rings. So if not three-membered as you can see, I was dealing with a 6-membered one here. This is usually the one we use. Okay? So if it's 4-membered, 5-membered, 6-membered, etcetera, you would use this naming system. Now, if it is a 3-membered ring, we could still use it but this is not going to be the most common way to name it. Okay?
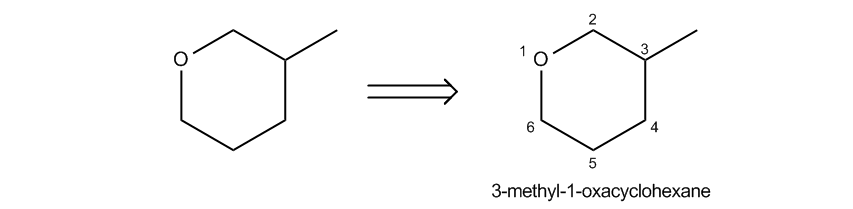
Epoxy Convention
Name as a typical alkane, and then include epoxy as a di-located substituent.
How to name epoxides using the epoxy convention.
Video transcript
If we are dealing with a 3-membered ring, there are much more common ways. One is the epoxy convention. So what the epoxy convention basically says is this: We have a substituent named an epoxy group. Okay? And we're just going to name our longest carbon chain as normal and then label the 3-membered ring as just the substituent coming off of that chain. And obviously, give it the lowest number. One other thing about this that's interesting is that you actually have to name the locations of both of the atoms that the three-membered ring is attached to. So as you can see here, my epoxide is going to get priority over the methyl, so I would choose this to be my first carbon over here. That means that my epoxy group, or my epoxy substituent, is across the 2 and the 3. Therefore, I'm actually going to call this a 2,3-epoxy substituent because I'm basically saying that I have a bond O across those two carbons. Then the rest of it, we're just going to name like always. So this would be 2,3-epoxy-5-methylhexane. Not so bad, right? Okay. So it's just something to consider that you could also use the epoxy convention. It's perfectly legit.
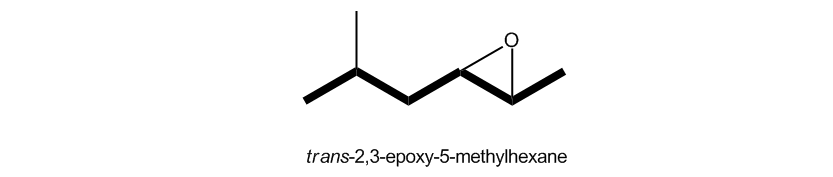
Oxide Convention
Name as an imaginary alkene, then follow with the word oxide.
How to name epoxides using the oxide convention.
Video transcript
Now there's on top of that, there's even one more way to name epoxides and that this one actually comes from even further back in the history. This one is actually like a reaction. Okay? What they're basically saying is name it as an alkene. So pretend that the epoxy wasn't even there, replace it with an alkene. Okay? Name it as the alkene. Entire name. And then at the end, just add the word oxide. Okay? Now how does that make sense? The reason that makes sense is because what we're saying is that we're basically assuming that we start off with a double bond and then we did an epoxidation to put an epoxide group on that double bond. Now, you might not know how to do that yet and that's fine. We're going to actually learn that pretty soon. But I'm just saying that this is almost coming from the reactivity side of things saying, well, I could start from a double bond and if I do an epoxidation, I could get an epoxide, so then it would call it an oxide of that double bond. So in this case, I would call this this would be hexene because I've got a 6 membered chain. Notice that my double bond would be across the 2 and the 3, but the way that I name double bonds is different from the way that I name epoxides. I actually don't say that this is a 2-3 alkene. I would just start where at the lowest number. So in this case, this is actually going to be what we call a 2-hexene. So don't get them confused. This would never be called a 2-3 hexene. You only do that for the epoxy substituent. So we know you have a 2-hexene. Now we need a substituent, the 5-methyl. Right? On the 5, so this would be 5-methyl-2-hexene oxide. Now just so you know, if your professor requires stereochemistry, if your professor is asking about stereochemistry, remember that's just like cis and trans and stuff, then you would have to provide it here. Okay?
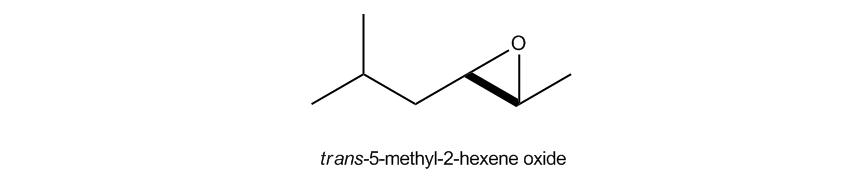
Note: I forgot to address in the videos that both of these would be trans due to the alkyl groups facing opposite sides of the ring.
Which of the following is the correct name of the following compound:

Which of the following is the correct name of the following compound:

Do you want more practice?
More setsHere’s what students ask on this topic:
What are epoxides and why are they highly reactive?
Epoxides, also known as oxeranes, are three-membered cyclic ethers characterized by significant ring strain. This strain arises because the bond angles in the three-membered ring are forced to be approximately 60°, which is much smaller than the ideal tetrahedral angle of 109.5°. This deviation from the preferred bond angles makes the ring highly reactive and prone to opening. The high reactivity of epoxides is utilized in various chemical reactions, making them important intermediates in organic synthesis.
 Created using AI
Created using AIHow do you name epoxides using the cycloalkane convention?
In the cycloalkane convention, the entire ring is named as if it were an alkane, based on its shape rather than the number of carbons. For example, a six-membered ring with one oxygen is named oxacyclohexane. The oxygen is given the highest priority and is assigned the number 1 position. Substituents are then numbered to give the lowest possible numbers. If there are multiple oxygens, prefixes like 'dioxa' are used. For instance, a six-membered ring with two oxygens would be named dioxacyclohexane.
 Created using AI
Created using AIWhat is the epoxy convention for naming epoxides?
The epoxy convention treats the epoxide as a substituent on the longest carbon chain. The three-membered ring is named as an epoxy group, and its position is indicated by the numbers of the two carbon atoms it is attached to. For example, if the epoxide is attached to carbons 2 and 3 of a hexane chain, it is named 2,3-epoxyhexane. The rest of the chain is named as usual, with other substituents given their appropriate positions and names.
 Created using AI
Created using AIHow do you name epoxides using the alkene oxide method?
In the alkene oxide method, the epoxide is named as if it were derived from an alkene. First, name the compound as an alkene, ignoring the epoxide. Then, add the word 'oxide' at the end. For example, if the epoxide is derived from 2-hexene, it is named 2-hexene oxide. If there are other substituents, they are named and numbered as usual. For instance, 5-methyl-2-hexene oxide indicates a methyl group on carbon 5 and an epoxide derived from 2-hexene.
 Created using AI
Created using AIWhat are the common names for three-membered cyclic ethers?
Three-membered cyclic ethers are commonly known as epoxides or oxeranes. These terms are synonyms and refer to the same structure, which is a three-membered ring containing one oxygen atom. The significant ring strain in these small rings makes them highly reactive, which is why they are often named as their own functional group in organic chemistry.
 Created using AI
Created using AIYour Organic Chemistry tutors
- Draw structures for the following: d. 2,3-epoxy-2-methylpentane
- Draw structures for the following: c. 2,2,3,3-tetramethyloxirane
- Write structural formulas for the following compounds. (g) cis-2,3-epoxyhexane (h) (2R,3S)-2-methoxypentan-3-o...
- Draw structures for the following:a. 2-propyloxiraneb. cyclohexene oxide
- Give two names for each of the following:a. <IMAGE>b. <IMAGE>
- Name the following: c. d.
- Give common names for the following compounds.(g) <IMAGE>
- Give IUPAC names for the following compounds.(g) <IMAGE>(h) <IMAGE>(i) <IMAGE>
- What is the major product obtained from the reaction of 2-ethyloxirane with each of the following reagents?a. ...

