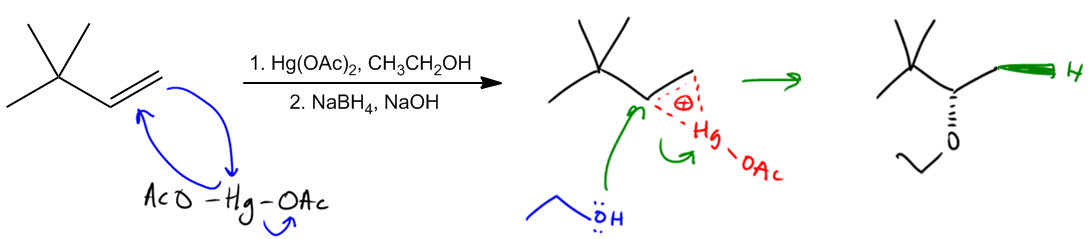
Another way that we can synthesize ether is through a reaction called alkoxymercuration. And you might immediately notice that this sounds a lot like another reaction that you're supposed to know by now and that's oxymercuration. So it turns out this is going to be the same exact mechanism as oxy oxymercuration or oxymerc except that in the top reagent in the oxymercuration step, we're going to use alcohol as our nucleophile instead of water. What you can see is that the biggest difference here is this right here. I have alcohol in place of the water and that's going to make an ether instead of an alcohol. Let me just walk you guys through this mechanism really quick.
So remember that what we get is Hg(OAc)2. And in the first step, what we do is the double bond attacks the mercury, kicks out one of the OACs and the mercury attacks back. So what we wind up making is a bridge, an ion bridge. So what that's going to look like is like this, where now I have a dotted partial bond to Hg. It's only attached to one OAC now. Dotted partial and a positive charge. There should be actually a dotted partial bond here too and a positive charge. So now we've got our intermediate. Notice that we can't get any shifts because there's no carbocation. And now this is the part that typically water would come in and attack which side? Do you remember? In this case, I have a Markovnikov site here and I have an anti-Markovnikov site there. Which one would it attack? Markovnikov because that's going to be the one with the most positive character. But instead of water, what's actually going to attack is, in this case, ethanol. So my ethanol attacks, kicks out the Hg, and what I wind up getting is something that looks like this. I'm going to try to fit it in here.
Where now, let's say that alcohol attacks from the bottom, then I would draw that the H