Now we're going to talk about one of the most common addition reactions in this entire section, and that's called halogenation. So let's just get right into it. Halogenation is the process of taking a double bond and adding a diatomic halogen, and at the end, what we're going to get is anti dihalides, specifically vicinal dihalides. Alright? So let's go ahead and just talk about the general features of this reaction.
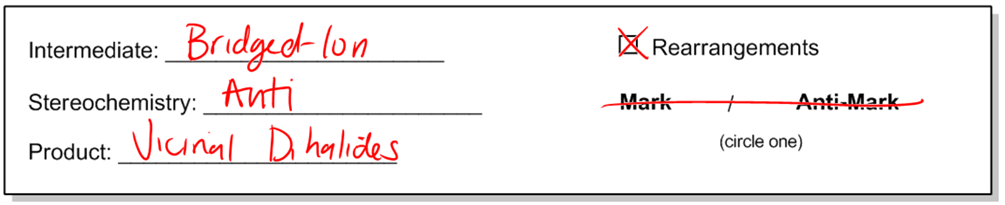
First of all, the mechanism is going to have an intermediate, and that intermediate is going to be similar to others we've learned. It's going to be a bridged ion. Okay? So maybe you can already start to visualize what that might be. Sorry about that. The stereochemistry is going to be anti. As I just showed you, you're going to get anti dihalides, so it would be anti. My product is going to be, like I said, vicinal dihalides.
Now, just to remind you, the word vicinal is used to mean that there are two things next to each other. So this relationship here would be vicinal because they are right next to each other. Vicinal is also the same as saying 1, 2 — basically, something on the one position and something at the two position, and that would be vicinal.
So, will there be rearrangements in this mechanism? No, there won't be because there's no carbocation. And then finally, since I'm adding two of the same thing, I'm not going to worry about Markovnikov's rule because I'm adding two of the same things, so it doesn't matter. So let's just scratch that out.
As you can see, the reaction looks pretty simple. We've got a double bond, so we know this is an addition reaction. We've got an electrophile that we're adding. Now notice that I have CCl4 down here. If you remember, this is actually going to be an apolar solvent. And apolar solvents are inert; they're not going to do anything, so don't even worry about it. That's just something that it happens to help the reaction, but it's not going to do anything. Another common solvent that you might see is like CH2Cl2. That just means it's the same thing, just instead of having four chlorines, you have two chlorines and two H's. Regardless, these are just solvents that don't do anything.
At the end, what we get is those vicinal dihalides. Let's go ahead and look at the reaction.