Alright guys, so at this point you're already pretty good at determining the equilibrium for an acid-base reaction if you have the pKa information. So I already taught you that you would compare the pKa of the acid and the conjugate acid. You would see which one is stronger and weaker, and that would determine your equilibrium. But what if you don't have pKa information? So what if you have an acid-base equilibrium question and there are 2 compounds that you just don't know the pKa values for? Or there are other situations too where pKa information might not be that helpful. And that's when we're going to use the factors affecting acidity. Okay? So for the next few pages, what I'm going to do is I'm going to introduce 5 factors that, even without knowing pKa values, we can still tell which one is going to be more acidic and less acidic based on these factors. So let's go ahead and get started. As I mentioned, there are 5 major factors, and we're really going to use these factors in 2 different situations. Okay? The first situation is that pKa information is unavailable for a molecule. So what that means is that maybe you didn't memorize it or maybe your professor didn't give it to you or maybe you did memorize it, and you just forgot it. Hey, if you're at a test and you don't remember it, it's unavailable. Alright? So then we might want to use factors affecting acidity. A second instance would be if the pKa's of 2 molecules are too similar to make a determination of highest acidity. So imagine if you're comparing 2 carboxylic acids. Well, they're both going to have a pKa of around 5, so how do I tell which one is more acidic and which one is less acidic? Well, with pKa information, you wouldn't really be able to do that. So we're going to need to look even more in-depth into these acids. Okay? So whenever we're analyzing these 5 different factors, what we're going to do is instead of looking at the acid, we're actually going to look at something else. That's going to be the stability of the conjugate base. Okay? How does this work? Okay. Well, the reason we look at the stability of the conjugate base is because that's going to tell us how willing the molecule is to give away a proton. So the more stable the conjugate, the more willing the acid is going to be to donate a proton. How does that make sense? Well, remember the conjugate base is what the acid becomes after it reacts. Okay? If the conjugate base is very, very stable, then it's going to say, hey, I'm fine giving up a proton as an acid. Because if I give up a proton, I'm just going to be really nice stable conjugate base. Awesome. But what if the conjugate base sucks? What if it's just like the worst conjugate of life? It's not going to want to exist very much. Okay? So instead, it's going to say, hey, I'd rather stay as the acid and have the proton on myself. So basically, the dissociation constant, the likelihood of me giving up a proton, is going to increase as my conjugate base becomes more and more stable. Does that make sense? And that's what these five effects have to do with. They are going to either increase the stability or decrease the stability of the conjugate base. Alright?
- 1. A Review of General Chemistry5h 5m
- Summary23m
- Intro to Organic Chemistry5m
- Atomic Structure16m
- Wave Function9m
- Molecular Orbitals17m
- Sigma and Pi Bonds9m
- Octet Rule12m
- Bonding Preferences12m
- Formal Charges6m
- Skeletal Structure14m
- Lewis Structure20m
- Condensed Structural Formula15m
- Degrees of Unsaturation15m
- Constitutional Isomers14m
- Resonance Structures46m
- Hybridization23m
- Molecular Geometry16m
- Electronegativity22m
- 2. Molecular Representations1h 14m
- 3. Acids and Bases2h 46m
- 4. Alkanes and Cycloalkanes4h 19m
- IUPAC Naming29m
- Alkyl Groups13m
- Naming Cycloalkanes10m
- Naming Bicyclic Compounds10m
- Naming Alkyl Halides7m
- Naming Alkenes3m
- Naming Alcohols8m
- Naming Amines15m
- Cis vs Trans21m
- Conformational Isomers13m
- Newman Projections14m
- Drawing Newman Projections16m
- Barrier To Rotation7m
- Ring Strain8m
- Axial vs Equatorial7m
- Cis vs Trans Conformations4m
- Equatorial Preference14m
- Chair Flip9m
- Calculating Energy Difference Between Chair Conformations17m
- A-Values17m
- Decalin7m
- 5. Chirality3h 39m
- Constitutional Isomers vs. Stereoisomers9m
- Chirality12m
- Test 1:Plane of Symmetry7m
- Test 2:Stereocenter Test17m
- R and S Configuration43m
- Enantiomers vs. Diastereomers13m
- Atropisomers9m
- Meso Compound12m
- Test 3:Disubstituted Cycloalkanes13m
- What is the Relationship Between Isomers?16m
- Fischer Projection10m
- R and S of Fischer Projections7m
- Optical Activity5m
- Enantiomeric Excess20m
- Calculations with Enantiomeric Percentages11m
- Non-Carbon Chiral Centers8m
- 6. Thermodynamics and Kinetics1h 22m
- 7. Substitution Reactions1h 48m
- 8. Elimination Reactions2h 30m
- 9. Alkenes and Alkynes2h 9m
- 10. Addition Reactions3h 18m
- Addition Reaction6m
- Markovnikov5m
- Hydrohalogenation6m
- Acid-Catalyzed Hydration17m
- Oxymercuration15m
- Hydroboration26m
- Hydrogenation6m
- Halogenation6m
- Halohydrin12m
- Carbene12m
- Epoxidation8m
- Epoxide Reactions9m
- Dihydroxylation8m
- Ozonolysis7m
- Ozonolysis Full Mechanism24m
- Oxidative Cleavage3m
- Alkyne Oxidative Cleavage6m
- Alkyne Hydrohalogenation3m
- Alkyne Halogenation2m
- Alkyne Hydration6m
- Alkyne Hydroboration2m
- 11. Radical Reactions1h 58m
- 12. Alcohols, Ethers, Epoxides and Thiols2h 42m
- Alcohol Nomenclature4m
- Naming Ethers6m
- Naming Epoxides18m
- Naming Thiols11m
- Alcohol Synthesis7m
- Leaving Group Conversions - Using HX11m
- Leaving Group Conversions - SOCl2 and PBr313m
- Leaving Group Conversions - Sulfonyl Chlorides7m
- Leaving Group Conversions Summary4m
- Williamson Ether Synthesis3m
- Making Ethers - Alkoxymercuration4m
- Making Ethers - Alcohol Condensation4m
- Making Ethers - Acid-Catalyzed Alkoxylation4m
- Making Ethers - Cumulative Practice10m
- Ether Cleavage8m
- Alcohol Protecting Groups3m
- t-Butyl Ether Protecting Groups5m
- Silyl Ether Protecting Groups10m
- Sharpless Epoxidation9m
- Thiol Reactions6m
- Sulfide Oxidation4m
- 13. Alcohols and Carbonyl Compounds2h 17m
- 14. Synthetic Techniques1h 26m
- 15. Analytical Techniques:IR, NMR, Mass Spect7h 3m
- Purpose of Analytical Techniques5m
- Infrared Spectroscopy16m
- Infrared Spectroscopy Table31m
- IR Spect:Drawing Spectra40m
- IR Spect:Extra Practice26m
- NMR Spectroscopy10m
- 1H NMR:Number of Signals26m
- 1H NMR:Q-Test26m
- 1H NMR:E/Z Diastereoisomerism8m
- H NMR Table24m
- 1H NMR:Spin-Splitting (N + 1) Rule22m
- 1H NMR:Spin-Splitting Simple Tree Diagrams11m
- 1H NMR:Spin-Splitting Complex Tree Diagrams12m
- 1H NMR:Spin-Splitting Patterns8m
- NMR Integration18m
- NMR Practice14m
- Carbon NMR4m
- Structure Determination without Mass Spect47m
- Mass Spectrometry12m
- Mass Spect:Fragmentation28m
- Mass Spect:Isotopes27m
- 16. Conjugated Systems6h 13m
- Conjugation Chemistry13m
- Stability of Conjugated Intermediates4m
- Allylic Halogenation12m
- Reactions at the Allylic Position39m
- Conjugated Hydrohalogenation (1,2 vs 1,4 addition)26m
- Diels-Alder Reaction9m
- Diels-Alder Forming Bridged Products11m
- Diels-Alder Retrosynthesis8m
- Molecular Orbital Theory9m
- Drawing Atomic Orbitals6m
- Drawing Molecular Orbitals17m
- HOMO LUMO4m
- Orbital Diagram:3-atoms- Allylic Ions13m
- Orbital Diagram:4-atoms- 1,3-butadiene11m
- Orbital Diagram:5-atoms- Allylic Ions10m
- Orbital Diagram:6-atoms- 1,3,5-hexatriene13m
- Orbital Diagram:Excited States4m
- Pericyclic Reaction10m
- Thermal Cycloaddition Reactions26m
- Photochemical Cycloaddition Reactions26m
- Thermal Electrocyclic Reactions14m
- Photochemical Electrocyclic Reactions10m
- Cumulative Electrocyclic Problems25m
- Sigmatropic Rearrangement17m
- Cope Rearrangement9m
- Claisen Rearrangement15m
- 17. Ultraviolet Spectroscopy51m
- 18. Aromaticity2h 34m
- 19. Reactions of Aromatics: EAS and Beyond5h 1m
- Electrophilic Aromatic Substitution9m
- Benzene Reactions11m
- EAS:Halogenation Mechanism6m
- EAS:Nitration Mechanism9m
- EAS:Friedel-Crafts Alkylation Mechanism6m
- EAS:Friedel-Crafts Acylation Mechanism5m
- EAS:Any Carbocation Mechanism7m
- Electron Withdrawing Groups22m
- EAS:Ortho vs. Para Positions4m
- Acylation of Aniline9m
- Limitations of Friedel-Crafts Alkyation19m
- Advantages of Friedel-Crafts Acylation6m
- Blocking Groups - Sulfonic Acid12m
- EAS:Synergistic and Competitive Groups13m
- Side-Chain Halogenation6m
- Side-Chain Oxidation4m
- Reactions at Benzylic Positions31m
- Birch Reduction10m
- EAS:Sequence Groups4m
- EAS:Retrosynthesis29m
- Diazo Replacement Reactions6m
- Diazo Sequence Groups5m
- Diazo Retrosynthesis13m
- Nucleophilic Aromatic Substitution28m
- Benzyne16m
- 20. Phenols55m
- 21. Aldehydes and Ketones: Nucleophilic Addition4h 56m
- Naming Aldehydes8m
- Naming Ketones7m
- Oxidizing and Reducing Agents9m
- Oxidation of Alcohols28m
- Ozonolysis7m
- DIBAL5m
- Alkyne Hydration9m
- Nucleophilic Addition8m
- Cyanohydrin11m
- Organometallics on Ketones19m
- Overview of Nucleophilic Addition of Solvents13m
- Hydrates6m
- Hemiacetal9m
- Acetal12m
- Acetal Protecting Group16m
- Thioacetal6m
- Imine vs Enamine15m
- Addition of Amine Derivatives5m
- Wolff Kishner Reduction7m
- Baeyer-Villiger Oxidation39m
- Acid Chloride to Ketone7m
- Nitrile to Ketone9m
- Wittig Reaction18m
- Ketone and Aldehyde Synthesis Reactions14m
- 22. Carboxylic Acid Derivatives: NAS2h 51m
- Carboxylic Acid Derivatives7m
- Naming Carboxylic Acids9m
- Diacid Nomenclature6m
- Naming Esters5m
- Naming Nitriles3m
- Acid Chloride Nomenclature5m
- Naming Anhydrides7m
- Naming Amides5m
- Nucleophilic Acyl Substitution18m
- Carboxylic Acid to Acid Chloride6m
- Fischer Esterification5m
- Acid-Catalyzed Ester Hydrolysis4m
- Saponification3m
- Transesterification5m
- Lactones, Lactams and Cyclization Reactions10m
- Carboxylation5m
- Decarboxylation Mechanism14m
- Review of Nitriles46m
- 23. The Chemistry of Thioesters, Phophate Ester and Phosphate Anhydrides1h 10m
- 24. Enolate Chemistry: Reactions at the Alpha-Carbon1h 53m
- Tautomerization9m
- Tautomers of Dicarbonyl Compounds6m
- Enolate4m
- Acid-Catalyzed Alpha-Halogentation4m
- Base-Catalyzed Alpha-Halogentation3m
- Haloform Reaction8m
- Hell-Volhard-Zelinski Reaction3m
- Overview of Alpha-Alkylations and Acylations5m
- Enolate Alkylation and Acylation12m
- Enamine Alkylation and Acylation16m
- Beta-Dicarbonyl Synthesis Pathway7m
- Acetoacetic Ester Synthesis13m
- Malonic Ester Synthesis15m
- 25. Condensation Chemistry2h 9m
- 26. Amines1h 43m
- 27. Heterocycles2h 0m
- Nomenclature of Heterocycles15m
- Acid-Base Properties of Nitrogen Heterocycles10m
- Reactions of Pyrrole, Furan, and Thiophene13m
- Directing Effects in Substituted Pyrroles, Furans, and Thiophenes16m
- Addition Reactions of Furan8m
- EAS Reactions of Pyridine17m
- SNAr Reactions of Pyridine18m
- Side-Chain Reactions of Substituted Pyridines20m
- 28. Carbohydrates5h 53m
- Monosaccharide20m
- Monosaccharides - D and L Isomerism9m
- Monosaccharides - Drawing Fischer Projections18m
- Monosaccharides - Common Structures6m
- Monosaccharides - Forming Cyclic Hemiacetals12m
- Monosaccharides - Cyclization18m
- Monosaccharides - Haworth Projections13m
- Mutarotation11m
- Epimerization9m
- Monosaccharides - Aldose-Ketose Rearrangement8m
- Monosaccharides - Alkylation10m
- Monosaccharides - Acylation7m
- Glycoside6m
- Monosaccharides - N-Glycosides18m
- Monosaccharides - Reduction (Alditols)12m
- Monosaccharides - Weak Oxidation (Aldonic Acid)7m
- Reducing Sugars23m
- Monosaccharides - Strong Oxidation (Aldaric Acid)11m
- Monosaccharides - Oxidative Cleavage27m
- Monosaccharides - Osazones10m
- Monosaccharides - Kiliani-Fischer23m
- Monosaccharides - Wohl Degradation12m
- Monosaccharides - Ruff Degradation12m
- Disaccharide30m
- Polysaccharide11m
- 29. Amino Acids3h 20m
- Proteins and Amino Acids19m
- L and D Amino Acids14m
- Polar Amino Acids14m
- Amino Acid Chart18m
- Acid-Base Properties of Amino Acids33m
- Isoelectric Point14m
- Amino Acid Synthesis: HVZ Method12m
- Synthesis of Amino Acids: Acetamidomalonic Ester Synthesis16m
- Synthesis of Amino Acids: N-Phthalimidomalonic Ester Synthesis13m
- Synthesis of Amino Acids: Strecker Synthesis13m
- Reactions of Amino Acids: Esterification7m
- Reactions of Amino Acids: Acylation3m
- Reactions of Amino Acids: Hydrogenolysis6m
- Reactions of Amino Acids: Ninhydrin Test11m
- 30. Peptides and Proteins2h 42m
- Peptides12m
- Primary Protein Structure4m
- Secondary Protein Structure17m
- Tertiary Protein Structure11m
- Disulfide Bonds17m
- Quaternary Protein Structure10m
- Summary of Protein Structure7m
- Intro to Peptide Sequencing2m
- Peptide Sequencing: Partial Hydrolysis25m
- Peptide Sequencing: Partial Hydrolysis with Cyanogen Bromide7m
- Peptide Sequencing: Edman Degradation28m
- Merrifield Solid-Phase Peptide Synthesis18m
- 32. Lipids 2h 50m
- 34. Nucleic Acids1h 32m
- 35. Transition Metals5h 33m
- Electron Configuration of Elements45m
- Coordination Complexes20m
- Ligands24m
- Electron Counting10m
- The 18 and 16 Electron Rule13m
- Cross-Coupling General Reactions40m
- Heck Reaction40m
- Stille Reaction13m
- Suzuki Reaction25m
- Sonogashira Coupling Reaction17m
- Fukuyama Coupling Reaction15m
- Kumada Coupling Reaction13m
- Negishi Coupling Reaction16m
- Buchwald-Hartwig Amination Reaction19m
- Eglinton Reaction17m
- 36. Synthetic Polymers1h 49m
- Introduction to Polymers6m
- Chain-Growth Polymers10m
- Radical Polymerization15m
- Cationic Polymerization8m
- Anionic Polymerization8m
- Polymer Stereochemistry3m
- Ziegler-Natta Polymerization4m
- Copolymers6m
- Step-Growth Polymers11m
- Step-Growth Polymers: Urethane6m
- Step-Growth Polymers: Polyurethane Mechanism10m
- Step-Growth Polymers: Epoxy Resin8m
- Polymers Structure and Properties8m
Ranking Acidity - Online Tutor, Practice Problems & Exam Prep
 Created using AI
Created using AIUnderstanding acidity involves several factors, including the stability of the conjugate base, which influences the acid's ability to donate protons. Key factors affecting acidity include the element effect, where electronegativity and atomic size play crucial roles; inductive effects from electronegative atoms not directly attached to the acidic hydrogen; resonance effects that stabilize conjugate bases through charge delocalization; hybridization effects related to s character; and steric effects that consider the size of substituents. These principles guide the comparison of acids and their strengths effectively.
Why is this section important? Because not all acid-base reactions are that easy.
When to Use Factors Affecting Acidity
Why we need factors affecting acidity and when to use them.
Video transcript
There are two situations in particular that making predicting equilibrium challenging:
- You don’t know the pKas of the acids (so how can you tell equilibrium?)
- The pKas for the acid and the conjugate acid are both the same (again, what do we do?)
No matter what, we know that the stronger acid will have the more stable conjugate base. Remember, reactivity and stability have that inverse relationship we talked about.
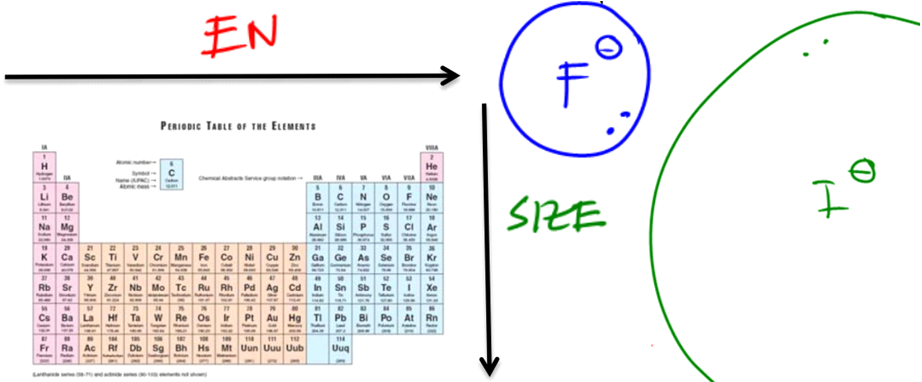
The Element Effect
This effect describes the way different atoms donate protons. For example C-H vs. N-H.
It consists of two trends:
- Electronegativity (EN) – the stronger the EN, the more stable the conjugate base will be with extra electrons.
- Size – The bigger (squishier) the atom, the less the conjugate base “feels” extra electrons.
Understanding the Element Effect.
Video transcript
Go ahead and look at the first and easiest one and that's the element effect. The element effect determines how loosely or strongly a particular element bonds with hydrogen. We can use these effects to compare different protonated elements to each other. For example, a perfect example of using the element effect would be having a nitrogen attached to an H and a sulfur attached to the H. Do we know the pKa of the nitrogen? Actually, yes. I taught you that the pKa of NH3 should be around 38. What about SH2? I actually didn't teach you that one. So then in this case, if I were to ask you which one is the most, the strongest acid, you would have really no clue on how to tell me because I never gave you the pKa of SH2. So that's why we have to use these factors.
It turns out that the element effect is going to consist of two trends, and the first one is electronegativity. Electronegativity just says that the stronger the electronegativity, the more willing the molecule will be to accept a lone pair as the conjugate. Here's the electronegativity trend, let's say HF, which is an H attached to the most electronegative atom, and then we have also CH4 over here. After each of these molecules gives up a proton, H+, it's going to turn into F- for the fluorine. This is the conjugate base. Now let's look at the conjugate base for the carbon, which would look like this, CH3-. Which of these conjugate bases is the most stable? It would be the fluorine since it is the most electronegative, so it's more comfortable having electrons on it compared to carbon. Does that first trend make sense so far?
Now let's look at our periodic table here. We were comparing nitrogen and sulfur. Just with the electronegativity trend, the sulfur would be the better acid, as even though you don't know the pKa of the sulfur, you know that the sulfur is more electronegative.
However, there's another effect that we need to know which is the size trend. What the size trend says is that the bigger or the squishier the atom is, the more willing it will be to accept a lone pair. Imagine F- versus a much bigger atom, I-. Because iodine is much bigger and more diffused, it can distribute the electrons over a larger space, making it a more stable conjugate base. Therefore, the stronger acid is HI, not HF. Hopefully, that makes sense to you guys in terms of the element effect. Just keep in mind that the element effect only has to do with hydrogens that are directly attached to different atoms. Now, take a break and try to solve this one and predict which one is going to be more acidic.
NOTE: This effect can only be used when comparing the way different atoms are attached to hydrogen. If you are comparing O-H vs. another O-H, it won’t work!
Without using pKa values, which of the following pair is more acidic?
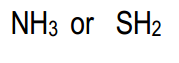
Using factors affecting acidity to rank acids
Video transcript
Hey everyone. So here it says without using pKa values, which of the following pairs is more acidic? Let's look at the first option. Here we have ammonia versus hydrosulfuric acid. Alright. We have H connected to nitrogen, and H connected to sulfur. Here we're going to try to figure out what type of technique we could use to determine which one is more acidic. Alright. In this case, we can rely on the element effect. If we look at our periodic table, we can see that nitrogen is in Group 5A. Sulfur is in Group 6A. But Sulfur is not in the same row as Nitrogen; it's one lower.
If we look at their electronegativities, although sulfur is more to the right than nitrogen, sulfur is not more electronegative. Sulfur, being lowered down, decreases your electronegative values. Nitrogen is just slightly more electronegative. But here the deciding factor in terms of the element effect is that Sulfur is larger in size. Remember, the trend is as we head towards the right side of the periodic table, our atomic radius or atomic size is going to decrease. But as you go down a group, it's going to increase. Sulfur being larger than nitrogen means that sulfur would be more acidic. Its bonds will be longer, and it'd be easier for us to remove an H+ from the sulfur atom. So here, SH2 would be more acidic than NH3. Again, it's based on our Element Effect or atomic size.
If we want to verify this, I know the question says without using pKa values, but we've decided on what our answer is. If we looked up the pKa values, you would see that NH3 has a pKa value of about 38. And Hydrosulfuric acid here has a pKa of around 10. It has a lower pKa value, which means it's more acidic. So here, Hydrosulfuric acid will be the more acidic of the pair, and it's based on the element effect or atomic size of sulfur versus nitrogen. Sulfur being larger means it's more acidic overall. Alright. Let's just use that one for this particular pair and continue with the other ones to see what the pairs will be for those.
Without using pKa values, which of the following pair is more acidic?
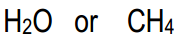
Using factors affecting acidity to rank acids
Video transcript
Alright, guys. So for this one, what we're looking at are the atoms that are directly attached to the hydrogen. So I would have a carbon here that I'm looking at, and I would have an oxygen here that I'm looking at because both of those are directly attached. And for these, both of them are in the same row. What that means is that carbon and oxygen have the same size, so the size effect isn't going to matter at all. Okay? But what is going to matter is electronegativity. And remember that electronegativity gets higher as we go to the right, so that means that water is going to have a higher, I mean, going to have a higher acidity than my methane (CH4). And remember, I said you can't use pKa values, you can just try to use the element effect. But if you wanted to double check, you could think about the pKa values. Remember the pKa of water is 16 and the pKa of CH4 would be around 50. Okay? So obviously water is going to be a much better acid. Alright? So let's move on to the next question.
Without using pKa values, which of the following pair is more acidic?

Using factors affecting acidity to rank acids
Video transcript
Alright. So for this one, what we had to look at is what are the atoms that are directly attached to the hydrogens. Okay? So in this case, I have an oxygen, but there are no hydrogens attached to it, so I have to look at the carbons next to it. And the carbons are what have the hydrogens on them. Okay? So I would have CH versus NH. Those are my 2 bonds that I am looking at. So then I would look at where those are in the periodic table. They happen to be right next to each other. Okay? So the size effect once again isn't really going to come into play, but the electronegativity is. This one is less electronegative and this one is more electronegative. So that means that the NH is going to be the one that is the strongest acid. Okay? So that means that it would be this one. And if you use pKa values, which again I told you not to use, but if you just wanted to confirm it, you would see that the pKa of this one is around 50 since it's just sp3CH and the pKa of this one is about 38. Alright. So now we're going to move on to our last one.
Without using pKa values, which of the following pair is more acidic?

Using factors affecting acidity to rank acids
Video transcript
Alright. So for this one, I'm going to take myself out of the screen so I don't cover it up. And, this one we already solved, but I just wanted to point out that the reason is because the HI has a larger size. So it's going to be more acidic than HF, even though F is more electronegative. Alright? Now, some of you might be wondering, why do I have to look at the conjugate base? Can't I just look at the acid? Because so far, I haven't been drawing any conjugate bases. I've just been looking at acids. Yes, you can. As long as you understand the reason why it's more stable is that the conjugate base is more stable. That’s why it's a better acid. Okay? So, hopefully, that made sense. Let's go on to our next factor that affects acidity.
The Inductive Effect
This effect describes the way that electronegative atoms that are NOT CONNECTED to the acidic proton make the conjugate base more stable.
Understanding the Inductive Effect.
Video transcript
Let's talk about the second factor that affects acidity, and that is inductive effects. So inductive effects describe the stabilizing properties that electronegative—write that down, electronegative—atoms that are not connected directly to acidic hydrogen have on the overall acidity. Now notice what I did to this word NOT CONNECTED, this phrase, I made it bold, I made it underlined, and I made it all caps. So do you think that's kind of important? Yeah. Right? It's very important. I need you to realize that this is kind of different from element effects. Element effects had to do with the atoms that are directly attached to the H. Okay? Whereas inductive effects have to do with atoms that are not attached to the H. That means they're on other parts of the molecule. They could be 2 carbons away, but they're still going to affect the acidity of the hydrogen. Okay? So that's the difference between element and inductive effects.
The way that works is that whenever a charge can be delocalized over more than one atom, that conjugate base or that charge is going to be more stable. So remember what's going on here. Remember that all of these acids, they're always going to give up a proton, and they're going to get a lone pair in return, and they're going to turn negative, and that's called the conjugate base. If I can spread out that negative charge over multiple atoms, that's going to make my molecule more stable. Alright? So the way that that works is that, by the way, delocalizing all that means is spreading out. Okay? So when I delocalize a charge, that means instead of it being in one place, I let it be in 2 or 3 places. I spread it out over an entire molecule.
Any EN force that helps to pull electrons away from the conjugate is called an inductive effect. If you can spread out that negative charge over multiple atoms, that base will be more stable.
Using electron clouds to understand the inductive effect.
Video transcript
So let's go ahead and look at this example here. Here I have 2 acids. Okay. So we're going to go ahead and draw some electron clouds in order to figure this out. But first of all, I just want to ask you guys, if you didn't know about this trend, could you solve this question with pKa's? Which one is the stronger acid? Could you solve it with pKa's? Actually, no, you couldn't because we said the pKa of alcohol is roughly 16. So this one is 16 and this is also an alcohol at 16. So according to my pKa rules, I cannot tell the difference of which one's more acidic and which one's less acidic. Alright? So this is one of those examples where my pKa's are too similar to tell the difference, so I'm going to need to use a factor affecting acidity.
Now check this out. Both of these have the same exact element effect because in both cases, I have an oxygen attached directly to the H. So is the element effect going to be different at all? No. They're the same. The electronegativity is the same and the size is the same because they're both oxygen. Okay? But what is different about these is that one of these has 3 fluorines really far away and then one of these has 2 fluorines really close. So let's see how that's going to affect it.
This is the part that I was talking about: electronegative things that are on other parts of the molecule will affect the H. So what I want to do is give away an H. Give away an H and what we're going to get now is the conjugate bases. Let's look at the conjugate bases. The conjugate bases look like this. Basically, a negative charge in the O here and a negative charge in the O here. The only difference is that when I draw, like if you want if I want to draw like an electron cloud of where those electrons are, for the O on the left-hand side, it would just look like this. Pretty much all those electrons would reside just on that O, meaning that these are localized.
But then check this one out over here. This one has these 2 very electronegative atoms right next to it. So what that means is that instead of all the electrons being around the O, some of them are going to get spread out over these fluorines. Okay? So what that means is that I'm going to have less of a charge around my O and I'm going to have a little bit more of a charge in other parts of the molecule. In fact, if I were to draw this again, I'd probably make this part around the O even a little bit smaller. It would probably look more like this, like more spread out like that.
Okay? Now you're never going to be asked to draw this. This is just my example. I'm just trying to get you guys to see how one of the conjugate bases is going to look different. But if you had to guess which of these conjugate bases is more stable, it would be the one on the right. It would be this one because this one is delocalized or spread out. Okay? So what that means is that if one of these acids, if both of these acids had the same opportunity to give up a proton, the one that would say, oh, me first me first would be the one on the right. Why? Because that's going to be the one that forms the more stable conjugate base right here. Whereas the other conjugate base, that one kind of sucks because it's all just in one place. Does that make sense, guys? Cool.
Factors that increase inductive effects:
- Strength of EN forces:-F > -Cl > -Br > -I
- Number of EN forces:The more the better
- Closeness of EN forces:The closer the better
The 3 factors that determine the strength of inductive effects.
Video transcript
So now what I want to talk about is what are the things that increase or decrease inductive effects. And there are actually three things that we want to look out for. Okay? So first of all, the strength of the electronegative entities. Okay. So the reason I'm using the word entities, I know that's like a weird word, is because it's not always going to be an atom. Sometimes it could be like a part of the molecule. Sometimes it could be like many atoms together. Okay? But anyway, the strength, that means that Fluorine is actually the best thing that you can use to have an inductive effect because Fluorine is the most electronegative atom. So that means if I were to rank the halogens in order, it would be Fluorine, then Chlorine, then Bromine, and then Iodine. K? Now check this out. This actually runs completely opposite to the element effect. Remember that in the element effect, the best halogen was iodine. Right? Because with the iodine, remember, it was really big and squishy and it could have a lot of electrons in it. But in this case, remember that the H is never directly attached to this halogen in inductive effects. In inductive effects, all I care about is which one is the most electronegative to take the most electrons away. Okay? So what that means is that actually, it's going to be the opposite. In this case, Fluorine is always going to be the best thing that you can have. Okay? Then the next thing is the number. The number just means the more the better. Okay? So if you have, like let's say that you have 3 Fluorines on one of them and 2 Fluorines on the other, the 3 Fluorines would win. Okay? And then finally, the proximity and that means the closer the better. The reason that proximity is important is because if your electronegative things are too far away, they're just not going to have an effect on it at all. And actually, that's what happened up far away, they're just not going to have an effect on it at all. And actually that's what happened in this conjugate base. This conjugate base had 3 fluorines, so you might have thought that it was going to actually be better. But it's so far away from my oxygen that it's not going to be able to have any effect on that electron cloud. Does that make sense? And the general rule is that if you are 3 carbons away or more, so 3 carbons or more or 3 atoms or more, then you would have no effect. Okay? So what that means is this would be 1, 2, 3. Anything after that is not really going to have an effect on the oxygen because it's just too far away. Alright. And I just realized that I was writing that off the page, so I'm just going to move that up a little bit so you guys can see that I said 3 atoms or more would equal no effect in terms of distance. Okay? So what that means is I want the strongest things, fluorines, more of them, as many as possible, and the closer the better. And that's going to be what makes my acid more acidic because it's going to stabilize the conjugate base more. Cool? Once again, a few of you guys might be asking me, but Johnny, can I just look at the acids instead of the conjugate? Yes, you can. But I need you to understand why it's more stable. And the reason has to do with these electron clouds. Okay?
Without using pKa values, which of the following pairs is more acidic?

Using Inductive Effect to determine acidity
Video transcript
Now what I'm going to do is I'm going to give you guys this problem that is actually really easy, but I'm still going to give it to you guys just as a free response. Go ahead and try to solve which one would be the more acidic, the right or the left. So go ahead and pause the video and tell me. So the answer is obviously this one would be more acidic. Okay? Because this one actually meets all three criteria. It has some electronegative elements that are not attached. It actually has 4 fluorines. It has a lot of them, 4, and they're all really close. They're all exactly on the alcohol. So this is an example of just a really easy question that you could get.
Now some of you guys might also be asking this, but Johnny, how about if my professor gives me one but not the other and if he switches them up? For example, what if on one of them, one of the acids has a bromine but then the other one has 2 bromines but then the other one has one fluorine. How do I know which one is better? And my answer to that is that professors are never that mean. So what they're going to do is because the only way to figure that out would actually be to do math and to actually do empirical calculations. So you're never going to have to worry about, oh, this one has more but this one is closer, stuff like that. Unless it's really obvious, like the one I gave you up there was obvious because it was way too far away. But if it was like you can't really tell the difference, you wouldn't get that kind of question. It's always going to be just these three things pulled together. So for example, one of them, let's say this was an alcohol, one of them could be like having 1 chlorine and then the other alcohol could be like having 1 fluorine. And then you just have to pick the difference between those 2. Okay. It wouldn't be like that you're having to pick the difference between 1 fluorine and 2 chlorines because that would be way too hard. Alright. So hopefully that makes sense to you guys. Let's go ahead and move on.
Resonance Effects
If a conjugate base is able to make a resonance structure, it will be more stable.
Understanding resonance effects. Which of the following –OH groups would be more acidic and why?

Understanding Resonance Effects
Video transcript
The next three factors aren't quite as complex, so I went ahead and grouped them into a category just called others. So let's go ahead and start with that third factor that affects acidity and that would be resonance effects. Alright? So resonance effects is actually just really easy. It's just whenever the donation of a proton leads to the formation of a possible resonance structure, okay, that conjugate base will be more stable. And the reason that the conjugate base will be more stable is because it can resonate. Remember that resonance structures enable for a charge to be in multiple places. That would make sense that it's delocalizing the charge. Alright? And that means that if the conjugate base is more stable, then the molecule will be a better acid. So check this out. For this example, I've given you two OH bonds. So I have OH and I have OH. So we're not going to use pKas to figure this out. It says which of the following pairs of acids would have the lower? But I'm not asking you to remember it, I mean even though you do, you should remember it, but I just want to use the factors affecting acidity to figure this out. So for both of these, do they have different element effects? Remember the element effect is the atom that's directly attached to the H. So in this case, they both have the same element effect. So I'm just going to write that here. Same element effect. Okay? And the reason is because they both have an oxygen attached to an H. That doesn't change. Alright? So the size, the electronegativity is exactly the same, but it turns out that one of these is a much better acid. In fact, one of these is called carboxylic acid and the other one is just an alcohol. So what is it about the carboxylic acid that makes it so much better as an acid? And it's the fact that let's look at the conjugate bases. The conjugate base for my carboxylic acid looks like this. O negative. Okay? The conjugate base, so I'm just going to put here as CB conjugate base. Alright? The conjugate base for my alcohol looks like this. Alright? So which of these is gonna be the one that's more stable? Well, both of them have a negative charge on the O, but notice that this O is stuck. This one, that negative charge isn't going to be able to go anywhere, so it's completely localized. Whereas on the other one, this one is actually going to be able to resonate using the two arrow rule that I taught you guys in resonance structures. So then I would make a bond there and I would break a bond there and I would actually get a new resonance structure that looks like this. And sorry it's a little bit crowded. I would get a new resonance structure that looks like that. So what that means is that I'm able to distribute this negative charge over those three atoms. Isn't that interesting? What that means is that one of them is going to be way more stable than the other and that is why carboxylic acid has a pKa of 5 whereas alcohol has a pKa of 16. That's a huge difference. Really, if it weren't for the carbonyl, that carboxylic acid would have a pKa of 16. But the carbonyl changes it so it can resonate. So now the pKa is basically like a trillion times better or like in terms of it's like a trillion times more acidic. Almost a trillion. Alright? So it's literally a way better acid. So that's called the resonance effect. Anytime you can make resonance structures, it comes into play.
Hybridization Effects
Understanding hybridization effects.
Video transcript
Now let's look at the hybridization effect. The definition of the hybridization effect is this: the higher the s character in an acid, the more stable the conjugate base becomes. We discussed s character when we talked about hybridization. Recall that 25% s character comes from sp3 hybridization, where you have one s and three p orbitals; therefore, 25% of the hybrid is s character. The s orbital is the closest to the nucleus and is the smallest, which means the more s character present, the closer the lone pairs are held to the nucleus. This proximity makes the conjugate base more stable by holding the electrons tighter to the positive, enhancing stability.
Now, considering the acidity trend, it would emerge that sp hybridization has 50% s character because it consists of one part s and one part p. Sp2 would have 33% s character, and sp3 would have 25% s character. Let's examine the pKa values to understand how this trend affects acidity. The pKa for an sp hybridized CH is 25. For sp2, characteristic of alkyne, the pKa is 44. And for sp3, typically found in alkane, it is 50. As the s character increases, so does the acidity. Does that make sense? When the s character proportion increases, the entirety of the hybrid orbital being s leads to greater acidity.
The higher the %s-character of the conjugate base, the more stable it will be.
- Recall, %s-character = (s-orbitals)/(total hybrid orbitals) x 100.
- Aka, sp3 = ¼ = 25% s-character
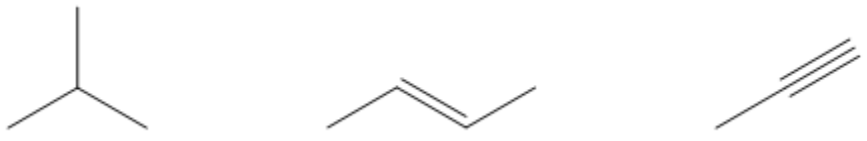
Which of the following hydrocarbons is the most acidic?
Which of the following hydrocarbons is the most acidic?
Video transcript
So this is just a way to explain that pKa trend. Which of the following hydrocarbons would be the most acidic? And you guys already know that it would be this one because this one would have an H there and that one would be sp hybridized, so it would have a pKa of 25. Is that cool?
Steric Effects
This rule really only applies with alcohols for now.
Understanding steric effects.
Video transcript
All right. So we get to our last one and this is called steric effects. Now I still haven't even talked to you guys about what sterics are, but that will be coming shortly. But basically, what steric effects say is that particularly with alcohols, this is really just going to deal with alcohols, the more easily solvated the conjugate base is, the more stable it will be. Alright, so what the heck does that mean? Solvated. Wow. That's a huge word. It just means how easily it will dissolve in an aqueous solution or how easily it will be mixed in an aqueous solution. All right? What that means is that we want our alcohols to not be very bulky in order to mix. If they are bulky, they're not going to have as easy of a time mixing into the solution. So the rule here that you just need to know is that the smaller the R group, the more acidic the alcohol. Oops. Acidic. Okay. So the smaller the R group, the more acidic the alcohol, going all the way down to water.
Let me show you an example. So basically, if I have HOH and then CH3OH and then let's say I just keep adding, I keep putting more and more groups. So let's say I had something like that. Okay, I have these 3 different these are not all alcohols. These 2 are alcohols and then the one on top of it is water. Okay? So just so you know, this first alcohol, that I have right here, let's say this is going to be, this would be like whatever. I'm just going to say this is just CH3OH. This first one would have a pKa of around 16. Okay? As I add R groups to it, so as I make R and R here, that pKa is going to start to go up because it's going to be a little bit less acidic. So in this one, we have a pKa of maybe around 17. Okay? It gets to the point where if you add enough R groups, the pKa can go all the way up to about 19 for terbutanol, which is one of the bulkiest alcohols. Alright? Then let's look at water. Water, instead of having an r group, it just has an H there. H is the smallest one of all. Remember I said smaller the R group? Well, H is really the smallest that it can get. Right? So that means that water actually has a pKa of 15.7, making it actually the best acid out of all of these. Why? Because it's the one with the smallest group next to the alcohol. Does that make sense? So basically, the smaller the bigger your group gets, the worse of an acid it gets. Okay? The smaller your group is, the better an acid. Okay? So, then the other trend is just the opposite of that. It would say the bigger that the R group is, the more basic the alkoxide. The alkoxide is just the name for the conjugate base. So that would just be anything that has an O negative on it. Okay?
Particularly with alcohols, the more easily solvated the conjugate base is, the more stable it will be.
- The smaller the R group, the more acidic the alcohol
- The bigger the R group, the more basic the alkoxide
Which of the oxides is the most basic?

Which of the oxides is the most basic?
Video transcript
Alright. So, yep, the one that would be the most basic would be this one right here. And the reason is because that's called Terbutoxide and it's the bulkiest base out of all of these that has the most R group sticking off of it, so it would be the one that is the worst solvated, so that means that it's going to be the least stable conjugate base, which means it's the most basic. Alright. So I know that was a lot of like it means this, which means this. Just remember the rules. If you're confused, just memorize these rules and you'll be fine. Alright? So now let's go ahead and do some practice.
Would the following reactions go to the right or the left? Draw the products and label ALL species. Provide the full mechanism.

Problem Transcript
Would the following reactions go to the right or the left? Draw the products and label ALL species. Provide the full mechanism.

Problem Transcript
Would the following reactions go to the right or the left? Draw the products and label ALL species. Provide the full mechanism.

Problem Transcript
Would the following reactions go to the right or the left? Draw the products and label ALL species. Provide the full mechanism.

Problem Transcript
Do you want more practice?
More setsHere’s what students ask on this topic:
What are the factors affecting acidity in organic compounds?
The factors affecting acidity in organic compounds include:
- Element Effect: Electronegativity and atomic size of the atom bonded to the acidic hydrogen.
- Inductive Effect: Stabilizing influence of electronegative atoms not directly attached to the acidic hydrogen.
- Resonance Effect: Stabilization of the conjugate base through charge delocalization.
- Hybridization Effect: Higher s character in the hybrid orbitals increases acidity.
- Steric Effect: Smaller substituents around the acidic hydrogen increase acidity by allowing better solvation of the conjugate base.
 Created using AI
Created using AIHow does electronegativity affect acidity?
Electronegativity affects acidity by influencing the stability of the conjugate base. A more electronegative atom bonded to the acidic hydrogen will stabilize the negative charge on the conjugate base better, making the acid more willing to donate a proton. For example, in the comparison between HF and CH4, the conjugate base F- is more stable than CH3- because fluorine is more electronegative than carbon, making HF a stronger acid than CH4.
 Created using AI
Created using AIWhat is the inductive effect and how does it influence acidity?
The inductive effect refers to the stabilizing influence of electronegative atoms that are not directly attached to the acidic hydrogen. These atoms pull electron density through sigma bonds, stabilizing the conjugate base. The strength of the inductive effect depends on the electronegativity of the atoms, their number, and their proximity to the acidic hydrogen. For example, a molecule with multiple fluorine atoms close to the acidic hydrogen will be more acidic due to the strong inductive effect of fluorine.
 Created using AI
Created using AIHow do resonance effects stabilize conjugate bases?
Resonance effects stabilize conjugate bases by delocalizing the negative charge over multiple atoms. This delocalization spreads out the charge, reducing the energy and increasing the stability of the conjugate base. For instance, in carboxylic acids, the negative charge on the conjugate base can resonate between two oxygen atoms, making the conjugate base more stable and the acid stronger compared to alcohols, where the negative charge is localized on a single oxygen atom.
 Created using AI
Created using AIWhat is the hybridization effect on acidity?
The hybridization effect on acidity is related to the s character of the hybrid orbitals. Higher s character means the electrons are held closer to the nucleus, stabilizing the conjugate base. For example, sp hybridized carbons (50% s character) are more acidic than sp2 (33% s character) and sp3 (25% s character) hybridized carbons. This is why alkynes (sp) are more acidic than alkenes (sp2) and alkanes (sp3).
 Created using AI
Created using AIYour Organic Chemistry tutors
- All of the following compounds can react as acids. Without using a table of acidities, rank them in order of i...
- Rank the following species in order of increasing acidity. Explain your reasons for ordering them as you do. ...
- Methyllithium (CH3Li) is often used as a base in organic reactions. b. What is the conjugate acid of CH3Li? Wo...
- Rank the ions (−CH3, −NH2, HO−, and F−) from most basic to least basic.
- a. Rank the following alcohols from strongest to weakest acid. CH2═CHCH2OH CH3CH2CH2OH HC≡CCH2OH
- Rank the following compounds from strongest to weakest acid: CH3CH2OH CH3CH2NH2 CH3CH2SH CH3CH2CH3
- If HCl is a weaker acid than HBr, why is ClCH2COOH a stronger acid than BrCH2COOH? [SOLVED]
- Which is a stronger base? a. or b. or
- Which is a stronger acid? c. CH3OCH2CH2CH2OH or CH3CH2OCH2CH2OH d. or
- The pKa of ascorbic acid (vitamin C, page 55) is 4.17, showing that it is slightly more acidic than acetic ac...
- Rank the following acids in decreasing order of their acid strength. In each case, explain why the previous co...
- The following compound can become protonated on any of the three nitrogen atoms. One of these nitrogens is muc...
- Choose the more basic member of each pair of isomers, and show why the base you chose is more basic. a. or ...
- The following compounds are listed in increasing order of acidity. In each case, the most acidic proton is sho...
- Choose the more acidic member of each pair of isomers, and show why the acid you chose is more acidic. a. or...
- The following compounds can all react as acids. CH3COOH CF3COOOH CF3CH2COOH < CH3CH2OH Rank the origi...
- 1. Consider the type of orbitals involved, and rank the following nitrogen compounds in order of decreasing ba...
- Consider each pair of bases, and explain which one is more basic. Draw their conjugate acids, and show which o...
- Write equations for the following acid–base reactions. Label the conjugate acids and bases, and show any induc...
- Ethanol, methylamine, and acetic acid are all amphoteric, reacting as either acids or bases depending on the c...
- Ethanol, methylamine, and acetic acid are all amphoteric, reacting as either acids or bases depending on the c...
- Which is a stronger acid? c. or d. CH3CH2CH2OH or CH3CH2CH2SH
- Which is a stronger acid? or
- For each of the following compounds, indicate the atom that is protonated when an acid is added to a solution ...
- For each of the following compounds, indicate the atom that is protonated when an acid is added to a solution ...
- Rank the carbanions shown in the margin from most basic to least basic.
- CH3CH2CH2COOH CH3CH2CHClCOOH ClCH2CH2CH2COOH CH3CHClCH2COOH Ka=1.52×10−5 Ka=1.39×10−3 Ka=2.96×10−5 Ka=8.9x10-...
- b. Explain the relative acidities. CH2═CHCH2OH CH3CH2CH2OH HC≡CCH2OH
- Using the table of pKa values given in [Appendix I] , answer the following: d. Which is more electronegative:...
- ••••) THINKING AHEAD Imidazole has two potentially basic sites (a and b). Of the two, where would you expect a...
- Rationalize the rather large difference in pKₐ values for the two carboxylic acids shown.
- Rank the following amines in order of their basicity (strongest base = 1 ; weakest base = 6).
- Which acid in each pair would you expect to more readily donate a proton to a basic compound? (b)
- Using qualitative reasoning for the acid–base reactions shown, (i) which is stronger, the acid or the conjuga...
- Using qualitative reasoning for the acid–base reactions shown, (i) which is stronger, the base or the conjuga...
- Identify the most stable conjugate base in each pair. Tell which structural features you analyzed and why you ...
- Identify the most stable conjugate base in each pair. Tell which structural features you analyzed and why you ...
- Identify the most acidic proton in each pair. Tell which structural features you analyzed and why you weighted...
- Identify the most acidic proton in each pair. Tell which structural features you analyzed and why you weighted...
- Which anion in each pair would you expect to react more quickly with H⁺ ? (a)
- Which anion in each pair would you expect to react more quickly with H⁺ ?(b) <IMAGE>
- Which is the most acidic compound in each pair? (a)
- Rank the indicated hydrogen in the following compounds from most acidic to least acidic:
- Which species in each of the pairs in Problem 80 is the stronger base? a. b.
- Which member of each pair is the stronger base? c. phenolate ion or ethoxide ion d. phenolate ion or acetate i...
- Which member of each pair is the stronger base? a. ethylamine or aniline b. ethylamine or ethoxide ion
- Which acid in each of the following pairs is stronger? e. f.
- Purine is a heterocyclic compound with four nitrogen atoms. a. Which nitrogen is most apt to be protonated? b...
- Which acid in each of the following pairs is stronger? c. d.
- For each of the following substituents, indicate whether it withdraws electrons inductively, donates electrons...
- Explain why the pKa of p-nitrophenol is 7.14, whereas the pKa of m-nitrophenol is 8.39.
- For each of the following substituents, indicate whether it withdraws electrons inductively, donates electrons...
- Which loses a proton more readily: a methyl group bonded to cyclohexane or a methyl group bonded to benzene?
- A nitro group (¬NO2) effectively stabilizes a negative charge on an adjacent carbon atom through resonance: T...
- (••) In each pair, choose the most basic compound. Justify your answer. (d)
- In each pair, choose the most acidic compound. Justify your answer. The most acidic proton in each compound ha...
- Which of the following indicated atoms would you expect to be most basic? (c) vs.
- Rationalize the difference in pKₐ values for the two hydroxyl groups.<IMAGE>
- Rank the compounds in each of the following groups from strongest acid to weakest acid: a.
- Explain why the a-hydrogen of an N,N-disubstituted amide is less acidic (pKa = 30) than the a-hydrogen of an ...
- Rank the compounds in each of the following groups from strongest acid to weakest acid: c.
- The pKa values of a few ortho-, meta-, and para-substituted benzoic acids are shown below: <s> The relat...
- Why is protonated pyrimidine (pKa = 1.0) more acidic than protonated pyridine (pKa = 5.2)?
- Why is the conjugate acid of morpholine more acidic than the conjugate acid of piperidine?
- Explain why pyrrole (pKa ~ 17) is a much stronger acid than ammonia (pKa = 36).
- Predict the products (if any) of the following acid–base reactions. (d) a-bromopropionic acid + sodium propio...
- Rank the compounds in each set in order of increasing acid strength. (c)
- Rank the compounds in each set in order of increasing acid strength. (b) CH3CH2CH2CHBrCOOH, CH3CH2CHBrCH2COOH,...
- Rank the compounds in each set in order of increasing acid strength. (a) CH3CH2COOH, CH3CHBrCOOH, CH3CBr2COOH
- Explain why the C-3 OH group of vitamin C is more acidic than the C-2 OH group.
- Explain the difference in the pKa values of the carboxyl groups of alanine, serine, and cysteine
- p-Nitrophenol (pKₐ = 7.2) is ten times more acidic than m-nitrophenol (pKₐ = 8.4) Explain. [This concept is th...
- Which proton, Hₐ or H_b, would you expect to have the lower pKₐ value?
- Which is the most stable base in each pair?(a) <IMAGE>
- Which is the most acidic compound in each pair?(b) <IMAGE>
- Which is the most acidic compound in each pair?(c) <IMAGE>
- Using qualitative reasoning for the acid–base reactions shown,(i) which is stronger, the base or the conjugate...
- Rank the following alcohols in order of descending pKₐ value. Explain your ranking.<IMAGE>
- Which of the following indicated atoms would you expect to be most basic?(b) <IMAGE> vs. <IMAGE>
- The nitrogen screened in purple in Figure 4.43 is not protonated at physiological pH. Why?<IMAGE>
- Which is the most stable base in each pair?(b) <IMAGE>
- (••) Identify the stronger base in each pair. Explain your choice. Citing pKₐ values is not an acceptable answ...
- Imidazole is a heteroaromatic base. Which nitrogen, a or b, is most basic?<IMAGE>
- Without using pKₐ values, pick out the least reactive (most stable) base in each pair. Explain your answer.(b)...
- Using qualitative reasoning for the acid–base reactions shown, (i) which is stronger, the acid or the conjugat...
- Identify the most stable conjugate base in each pair. Tell which structural features you analyzed and why you ...
- Identify the most stable conjugate base in each pair. Tell which structural features you analyzed and why you ...
- Identify the most acidic proton in each pair. Tell which structural features you analyzed and why you weighted...
- Identify the most acidic proton in each pair. Tell which structural features you analyzed and why you weighted...
- Which acid in each pair would you expect to more readily donate a proton to a basic compound?(a) <IMAGE>
- Why is the value of protonated hydroxylamine (6.0) so much lower than the value of a protonated primary amine ...
- (••) In light of your answers to Assessments 24.54 and 24.55, rank the following based on the rate of protonat...
- (•••) Consider the following drugs used to treat the indicated diseases. Would you expect the activity of thes...
- Which is the most stable base in each pair?(c) <IMAGE>
- Explain why a base can remove a proton from the a-carbon of N,N-dimethylethanamide but not from thea-carbon of...
- Citrus fruits are rich in citric acid, a compound with three COOH groups. Explain the following:a. The first p...
- Which of the following amino acid side chains can help remove a proton from the a-carbon of an aldehyde?<IM...
- Rank the following compounds from easiest to hardest at removing a proton from its methyl substituent:<IMAG...
- CCl3CH2OH CH2ClCH2OH CHCl2CH2OHKa=5.75×10−13 Ka=1.29×10−13 Ka=4.90×10−13b. Explain the relative acidities.
- Glycine has pKa values of 2.3 and 9.6. Do you expect the pKa values of glycylglycine to be higher or lower tha...
- Fosamax (shown on the previous page) <IMAGE> has six acidic groups. The active form of the drug, which h...
- e. Which has a greater Ka: cyclohexylammmonium ion or anilinium ion?f. Which is a stronger acid: cyclohexylami...
- The pKa values of a few ortho-, meta-, and para-substituted benzoic acids are shown below:<IMAGEs>The re...
- b. Explain why, when the guanidino group of arginine is protonated, the double-bonded nitrogen is the nitrogen...
- Which compound is the strongest base?<IMAGE>
- Consider the following compounds that vary from nearly nonacidic to strongly acidic.Draw the conjugate bases o...
- Choose the more acidic member of each pair of isomers, and show why the acid you chose is more acidic.d. <I...
- Choose the more acidic member of each pair of isomers, and show why the acid you chose is more acidic.f. <I...
- The pKa of ascorbic acid (vitamin C, page 55) <IMAGE> is 4.17, showing that it is slightly more acidic t...
- Choose the more basic member of each pair of isomers, and show why the base you chose is more basic.c. <IMA...
- Choose the more basic member of each pair of isomers, and show why the base you chose is more basic.e. <IMA...
- The following compounds can all react as bases.CH3CH2NH2 <IMAGE>CH3CONH2 <IMAGE>NaOHCH3CH2OH <I...
- Four pairs of compounds are shown. In each pair, one ofthe compounds reacts more quickly, or with a more favor...
- Use resonance forms of the conjugate bases to explain why methanesulfonic acid (CH3SO3H, pKa = - 2.6) is a muc...
- Acetylacetone (pentane-2,4-dione) reacts with sodium hydroxide to give water and the sodium salt of a carbanio...
- (••) Identify the stronger base in each pair. Explain your choice. Citing pKₐ values is not an acceptable answ...
- (••) Identify the stronger base in each pair. Explain your choice. Citing pKₐ values is not an acceptable answ...
- (••) Identify the stronger acid in each pair. Explain your choice. Citing pKₐ values is not an acceptable answ...
- Without using pKₐ values, pick out the least reactive (most stable) base in each pair. Explain your answer. ...
- Without using pKₐ values, pick out the least reactive (most stable) base in each pair. Explain your answer. ...
- Which of the following indicated atoms would you expect to be most basic? (a) vs.
- Without using pKₐ values, pick out the more acidic compound in each pair. Explain your answer. (a)
- Without using pKₐ values, pick out the more reactive (least stable) base in each pair. Explain your answer. ...
- Tenormin, a member of the group of drugs known as beta-blockers, is used to treat high blood pressure and impr...
- A carboxylic acid ( pKₐ = 5) is 10₁₁ times more acidic than an alcohol. Why? (pKₐ = 16.)<IMAGE>

