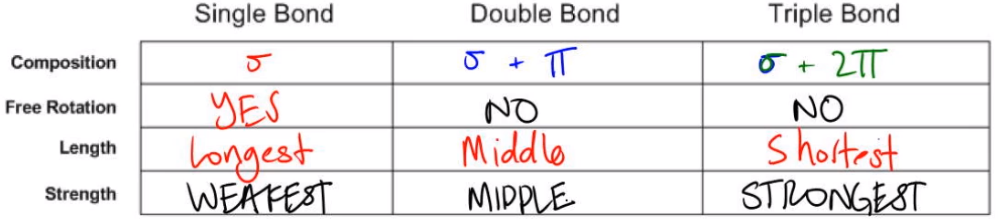
So basically, we have three types of bonds: single, double, and triple. And what we need to know is just a few facts about each. The first thing is composition. Composition deals with whether they are made out of single bonds, pi bonds, a combination? We need to know this. So, I already told you guys what single bonds were. Do you guys remember? It's just made out of one sigma. Okay? So whenever I say sigma, it means single. Single means sigma. They're the same thing when it comes to just if it's just one sigma, that's a single. Now double and triple get a little bit more complicated because remember that I said it's actually more than one orbital combining. So for a double bond, it's actually going to be one sigma as always because there's still one region of overlap in the middle from the s orbitals. Okay? But there are also these p orbitals, and the p orbitals have two regions of overlap. So that means that a double bond is made out of a pi and a sigma or a sigma and a pi. Alright? So overall, that kind of makes sense because a double bond has two orbitals that are overlapping. Alright? And then finally, a triple bond, you could guess. Now, this one's going to have three orbitals overlapping. What that's going to be is still that one sigma from the middle from the s's. But for a triple bond, now you have two sets of p orbitals on both sides that are overlapping, one up and down and one side to side. So what that means is that a triple bond is made out of one sigma and two pi. Alright? Cool. So that's the first thing.
Now I want to talk about free rotation. Why? Because this has a lot to do with composition. So remember that I said that a bond is just a region of shared space with electrons. So if I just have one interaction in the middle, where they're overlapping, is it possible to rotate one of the atoms like I'm doing right now and keep the bond intact? And the answer is yes. If I rotate this atom, I can still keep this bond together. Okay? So it turns out that for a single bond, since they only have one region of overlap, we're just going to write a big yes right here. Yes, I have free rotation there. Okay? Because I can rotate one of the atoms as much as I want. It's always going to have that bond present. Now let's look at double bonds. Double bonds, they do have that region of overlap in the middle, that one area. But they also have a region at the top and the region at the bottom from the pi bonds. So it's going to look something like this, where you have basically electrons overlapping at the top, electrons overlapping at the bottom. If I try to rotate one of the atoms, is that going to break or change my bond at all? And, actually, yes, it will. What's going to happen is that this top orbital or this top region is going to break, and this bottom region is going to break. That's really unfavorable and really unlikely to happen. The reason is because remember that I said that when you form bonds, that saves a ton of energy. So what that means is that I'm going to have to spend a lot of energy in order to rotate and break this bond. And that's energy that these atoms don't have most of the time. Alright? So what that means is that these, double bonds are not going to be able to rotate. And, actually, that's what I want you guys to write for double and triple. They're both not going to be able to rotate because of the fact that if you rotate them, you're going to have to break them, and it's very difficult to break the bonds just to rotate. Okay? So good. Now you guys understand the only ones that rotate are the single bonds.
Now let's talk about length. Hopefully, you guys should remember what I talked about with sigma versus pi. Which one was the longer one? Do you guys remember? Sigma is the longest. So the single bond would be my longest. It actually turns out that because the triple bond is made out of one sigma and two pi, this one's going to be the shortest. Okay? And then you could guess that the double bond is going to be like the middle child. Alright? So just the middle. Going to be in the middle, not quite as long, but not quite as short. Alright?
Now let's go on to strength. So I already talked to you guys about this earlier. Which of these is going to be the weakest overall in terms of single, double, or triple? And the answer is that the single bond is the weakest. Okay? The reason for that is that there's only one region of overlap that is saving energy. Okay? Now the triple bond is actually going to be the strongest. Okay? The reason for that is that it has three orbitals that are overlapping, and each one is saving a little bit more energy. And that means that once again, my double bond is going to be in the middle. So now I want to ask you guys a question though. Which of the orbitals is the strongest? Is the sigma bond the strongest or is the pi bond the strongest? The answer is that the sigma bond is the strongest because it saves the most energy of the three bonds for the triple.