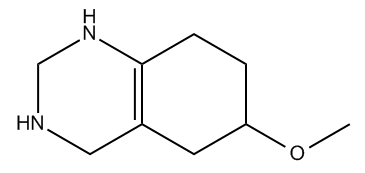
Hey guys. So now we're going to talk about a topic called index of hydrogen deficiency, and this is a topic that usually isn't covered in the first chapter of most textbooks. So you're probably thinking, "Johnny, why are you teaching this to me if I don't need to know it yet?" Well, because there's this other topic called constitutional isomers, and that topic is much easier to understand if you already know the IHD rules. So what I'm going to do here is jump a little bit forward and teach you something that you don't need to know yet because it's going to make isomers so much easier to understand for this chapter. So let's go ahead and begin.
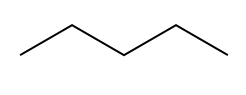
There are actually two different ways to compute the IHD, and that is one structural and the other one is with the molecular formula. What I'm going to do on this page is just show you the structural one, which is actually the easier one, and then we'll move on to the molecular formula. So first of all, let's just talk about what index of hydrogen deficiency is. Well, what it has to do with is how unsaturated or saturated a molecule is. So what's that word saturated? Well, a saturated molecule is any molecule that has as many hydrogens as possible.
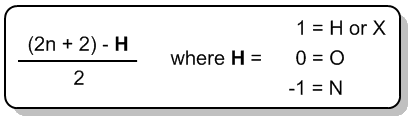
There's this formula that we can use in all chemistry to figure out if it has as many hydrogens as possible, and that formula is, maybe you remember it from general chemistry, It's n=2n+2, where n is equal to the number of carbons. So I have my 2n+2 rule, and what that's going to do is it's going to predict for me how many hydrogens I need for my structure for it to be fully saturated, meaning it has as many hydrogens as it can possibly hold. Any molecule that has less than that number, 2n+2, is going to be considered to be unsaturated. And these are words, saturated and unsaturated; they might sound familiar to you, and that's because these are words we use actually in cooking.
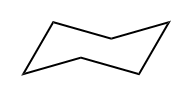
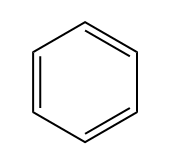
So, saturated fat would actually be a fat. Fats are these long carbon chains. There would be this fat that has as many hydrogens as possible. Okay. An unsaturated fat would be one that's missing a few hydrogens. So you might be wondering, "Okay, Johnny, what makes carbons miss hydrogens? Why would you be missing some?" It turns out it only happens for two different reasons. Okay? So I'm going to write this down. It happens for pi bonds. Remember that pi bonds just have to do with double bonds or triple bonds; they both have pi bonds. And then also rings. Rings are also going to take away some of the hydrogens from a molecule because what that means is that the end that one of the ends is supposed to be CH3 and the other end is supposed to be CH3. When you make a ring, they have to fuse together, so that means that now you have to make them CH2s. So what I'm trying to say is that a ring actually takes some hydrogens away from the carbon structure.