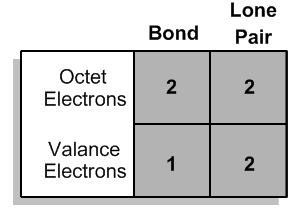
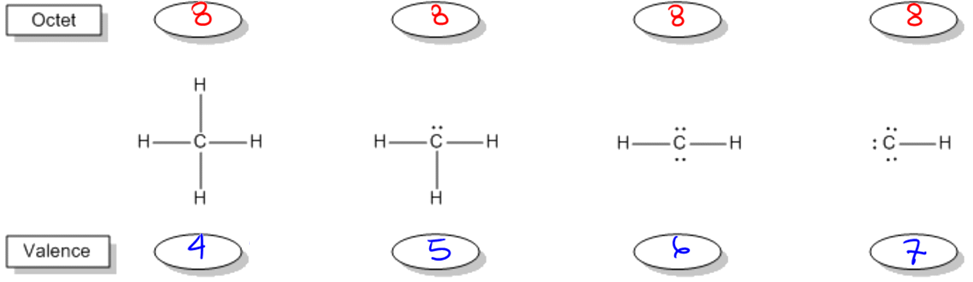
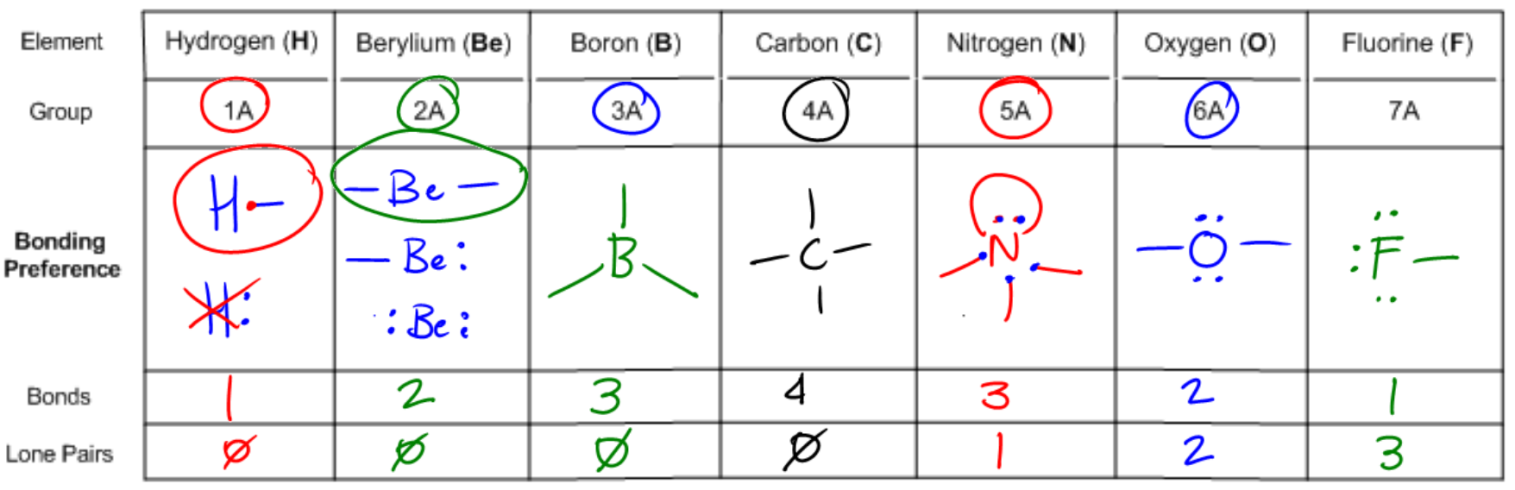
It turns out that if your octet is filled with all these atoms, the way you determine the one that's most stable is by figuring out what row that atom is in. It turns out that for each of the second row elements, the element is going to prefer to own or it's going to prefer to have a valence determined by its group number. So what that means is that if an atom is in group 4, like carbon, then how many valence electrons is it going to want to have? 4. So this is going to be the most stable arrangement of carbon by far, whereas all these other ones are going to get progressively worse and worse. In fact, some of these don't even exist because they would be so crazy hard to create. They'd be so unstable. Does that make sense? So now what I want to do is extend this because you're like oh, well, Johnny, everyone knows carbon has 4 bonds. Maybe you learned that in gen chem. But now I want to extend this to all the other atoms so that you guys will know for the second row what they look like and what they're always going to want to look like in their neutral bonding preference. All right. So let's go through this really quick. As you can see, I've listed different atoms kind of in order of where they are in the periodic table and we're just going to go ahead and start off with hydrogen. Now I understand that hydrogen is not in the 2nd row, but it's way more common than lithium, so we're going to just work with hydrogen. We're going to replace that one. Hydrogen is in group 1a and that means that how many electrons does it want to have in its octet? Do you guys remember? 2. So that means that how can an atom have an octet? Well, it can either have lone pairs or it can have bonds. So that means the hydrogen can exist in 2 different forms. I could have a hydrogen that has a bond. That would give it its 2 electrons. It would fill an octet, but I could also have a hydrogen that has a lone pair. Do you guys see that? Would both of these fulfill the octet? Yes. They would both fulfill the octet. So now my question is, are both of these equally stable? The way that I find that out is I look at the group number. Well, what's the group number? 1. So how many valence electrons does the first hydrogen have? It has one because I only count one for each stick. The second one, how many does it have? 2. So which one is going to be the most stable? This is going to be the way that hydrogen looks. It will not look like this. That's why every time you see hydrogen, it's always attached with just one stick, with one bond because that's going to be the one that satisfies not only the octet but also the valence of having one stick or one valence electron. Does that make sense? Cool. So now what I want you guys to do down here, now that we've figured that out, is really just write what we have. Like let's write our results. How many bonds does hydrogen like to have? 1. How many lone pairs does it like to have? 0. You guys cool with that? Let's move on. So now let's go to beryllium. So beryllium, do you guys remember how many octet electrons beryllium likes to have? Four. All right. It likes to have 4. So there's going to be actually 3 different ways that we could do this. I'll draw all 3 just so that you guys can kind of realize what that is. We could have beryllium with 2 sticks. We could have beryllium with a stick and a lone pair. Or we could have beryllium with 2 lone pairs. Are you guys getting that? All of these versions would satisfy the octet rule equally. But the way I determine the ones that's most stable is I look at the group number. What's the group number? 2. If I'm in group 2, which of these is going to be the favored arrangement? You guys got it. It's beryllium with the 2 sticks. Because that's the only one that is going to not only fulfill the octet but also fulfill the valence. So that means that beryllium in its bonding preference likes to have how many bonds? How many lone pairs? 0. Okay. So hopefully, that's making sense. Let's move on to the next one. All right. So now we are at boron. Do you guys remember how many electrons, octet electrons boron likes to have? This is from your octet rule. I think we talked about it. 6. How many valence electrons does it like to have? 3. So can you guys predict what I'm going to want to do here? I think I heard it. What you're going to want is boron with 3 bonds. The reason is because that's not only going to fulfill your octet of 6, but it's also going to fulfill your valence of 3. Cool? So that means that now it's going to want to have 3 bonds and 0 lone pairs. Are you guys kind of seeing a pattern here? None of these want lone pairs and they're escalating. So we already got to carbon and just by looking at this pattern, what should the next number be? Well, it should be that I have 4 bonds and 0 lone pairs. And when you draw it out, it actually does come out to that. This carbon wants to have 8 electrons. Remember that carbon, nitrogen, oxygen, and fluorine all want to have 8. So they're all going to stay at 8 and it wants to have 4 valence electrons because it's in group 4. So that actually is the arrangement. So now, let's continue to apply wants to have It wants to have 8. If we were just to apply this pattern, don't draw this yet, but I'm just showing you, what I would do is I would draw a 5 here and a 0 here and I would say nitrogen wants to have 5 bonds and 0 lone pairs. Is there a problem with that? Am I making a mistake? I'm actually making a huge mistake. The reason is that if nitrogen had 5 bonds, would it still satisfy the octet rule? No because remember, each bond counts as 2. So if nitrogen had 5 bonds, it would have 10 electrons and it would break the octet rule and it would be even worse off. It would be super unstable. So that means that somehow I need to get nitrogen to have 5 valence electrons, but still only 8 octet electrons. So that means that out of these electrons, out of these 8 electrons, I need to get nitrogen to own more of the electrons. How can we get it to own more? By adding lone pairs. Hopefully, what you guys maybe came to on your own is that nitrogen, what I'm going to want to do is 3 bonds and one lone pair. Because if I can do that, then what I'm going to get is I have 8 electrons total. 8. And then I have every stick counts as 1. 1, 2, 3. And every dot counts as 1. 4, 5. So now when I go down to my pattern, it's actually going to be that I want 3 bonds and one lone pair. Now this is going to start a new pattern. So for oxygen, oxygen still wants 8 electrons, but now it wants to own 6 of them. It wants even more because it's in group 6. So can you guys think of an arrangement that would work for that? Yeah, you guys are smart. You guys know what's going on now. So it would be 2 bonds and 2 lone pairs. Because now the more lone pairs I have, the more I'm owning. So that means that now this would count as 6 valence electrons, 8 octet, so this would be 2 bonds and 2 lone pairs. You guys can see the pattern here. We can guess what the last one's going to be and that pattern actually does hold. Fluorine would want to have you can see the bonds keep decreasing, so fluorine would have one bond and it would have 3 lone pairs. That actually makes sense because if you give fluorine one bond and 3 lone pairs, what you get is an octet of 8, but a valence of 7. Now guys, I really can't emphasize the value of understanding this enough because these bonding preferences are what a bunch of other topics are built off of. For example, bond line structures, formal charges, all this stuff has to do with your knowledge of being able to know these preferences. Also, it's just going to help you draw compounds later.