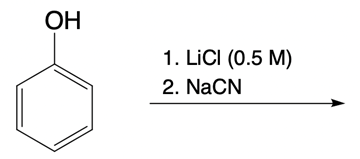
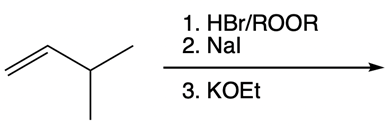
Hey, everyone. In this video, let's take a look at nucleophilic catalysis. Now, in Orgo 1, we learned that double SN2 caused retention of R and S configurations, but it also serves as a nucleophilic catalyst. When I say nucleophilic catalyst, I mean it displaces a leaving group with a better nucleophile/leaving group. The general trend of the periodic table is as we head down a group, our atomic size is supposed to increase.
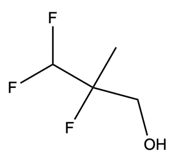
And when it comes to group 7A or halogens, this is the trend that we see. As we go down the group, we can see that the halogens get larger until we get down to iodine. Now, this would mean that iodine would be a better nucleophile and leaving group than bromine, chlorine, and fluorine. Bromine would be a better nucleophile and leaving group than chlorine and fluorine, etcetera. Now, if we take a look at the mechanism here, we're going to say that this blue star here means that that is a chiral center.
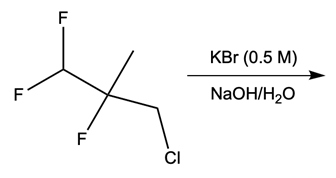
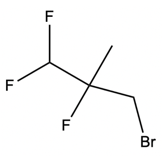
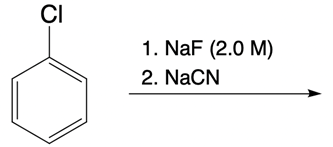
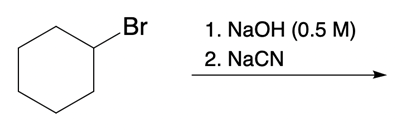
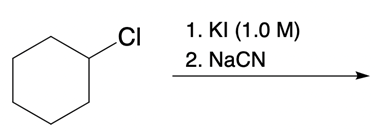
And we're going to start out with this alkyl halide. That halogen will be displaced by a larger halogen, which represents a better leaving group, a better nucleophile. And remember, because this is happening by an SN2 mechanism, we have inversion of our configuration. We go from wedged to dashed. So here we have inversion going on.
But remember, we're doing double SN2 so it's going to happen again. We're going to use now another nucleophile to come in and attack here and kick this halogen out. Now, that halogen is kicked out and leaves even better than our first halogen because it's larger, it's lower down group 7A. Now, hitting it again causes the bond to flip again. So we start out with wedged and we're ending with wedged.
So, in essence, we have retention going on. Right? So we inverted twice, they'll end up with the same type of bond we had at the end. This is the beauty of double SN2. But now, we're also seeing that replacing the first halogen with a better halogen means that this second SN2 would happen that much more quickly.
We've increased the rate of this exchange. And as we click on to the next series of videos, we'll see how this happens numerically. What is the numerical value? What is the increase in rate if we could somehow calculate it? Alright.
So just keep that in mind when we're talking about nucleophilic catalysis.