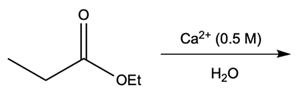
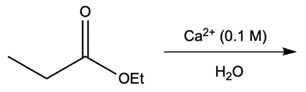
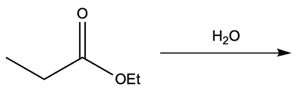
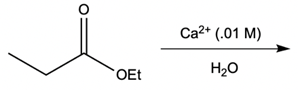
Hey everyone, in this video let's take a look at metal ion catalysis. Now, here we're going to talk about the different types of metal ion catalysis that exist. We're going to say metal ions, typically those with a 2+ or 3+ charge, act as catalysts by forming complexes with the lone pair of an electron-rich atom. We're going to say the metal ion can increase the rate of reaction by two factors, and those two factors are metal ion coordination and water activation. With metal ion coordination, we're going to say the metal ion acts like a proton, kind of like an H+ ion, and it does it in one of two ways.
It makes the carbonyl carbon more reactive towards nucleophilic attack or it stabilizes the leaving group once it's kicked out. Let's take a look at 1a. Here we have a carbonyl group and we have our metal ion. We're not designating it as either plus 2 or plus 3. We're going to say here that the metal forms this bond with the oxygen.
We represent this bond as this dashed bond here. The metal will have a partially positive charge and the oxygen, in essence, is making three connections, two to the carbon, one to the metal, so it'd be partially positive as well. This opens up the carbonyl carbon to nucleophilic attack. So the nucleophile will come in, hit here, and kick this bond up. In 1b, we're talking about the stabilizing effect that the metal ion can have on the leaving group.
So here, this OEt group forms its connection to the metal. Here we'd say, as a result of this, the oxygen is partially positive just like this metal. It is now a better leaving group when the OEt is attached to the metal. So this oxygen decides to make a double bond here, kicking it up. Now, water activation, we're going to say here that the metal ion increases the rate of hydrolysis of water, turning it into a stronger nucleophile.
When we talk about hydrolysis, we're talking about the splitting of water into its H+ and its hydroxide ion components. Here we're going to say based on the equilibrium arrows, the water molecule is more greatly favored, but all that matters is that we're making a small trace amount of this metal hydroxide complex. Here we have our metal. It's going to form a connection with the hydroxide. The metal would still be partially positive.
The hydroxide partially negative. This metal hydroxide complex is what we could utilize in some of these reactions, these nucleophilic reactions because under physiological pH, which is around 7.4, you can't really have a full hydroxide ion. So, the next best thing would be this metal hydroxide. So, in biological systems, it's important to be able to do this water activation. It allows us to change the less nucleophilic water molecule into the more nucleophilic metal hydroxide complex.
We can utilize that to do nucleophilic addition, nucleophilic substitution reactions later on. Alright. So again, these represent our different types of metal ion catalysis that exist. 1a and 1b are similar because the metal is acting as a proton. And then in b in 2, we're basically water activating. We're changing our water into a more reactive metal hydroxide complex.