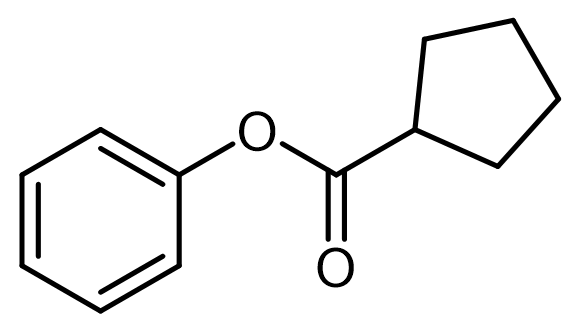
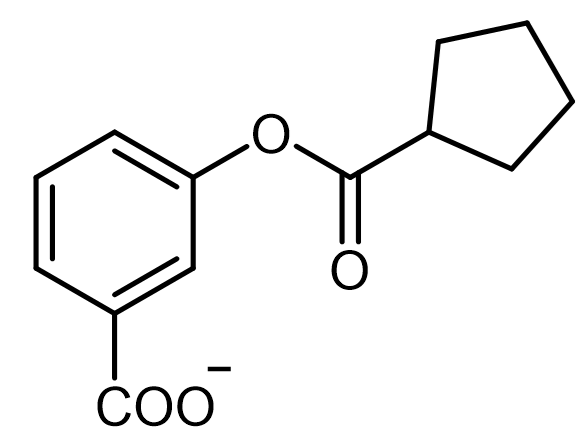
Everyone, in this example, it asks to write a plausible mechanism for the following hydrolysis reaction. Here, we have our carboxylate anion that's attached to the same structure as the ester, and at the end, we're going to split the ester bond to create our alcohol and our carboxylic acid. Now, step 1 deals with deprotonation and nucleophilic attack. Here we're going to say that the carboxylate anion deprotonates water to produce our hydroxide ion.
So, this oxygen will deprotonate the water. This oxygen holds on to the electrons. The hydroxide ion attacks the carbonyl group to form a tetrahedral intermediate. So, it would use its lone pair to attack here, keeping this bond here. As a result, what are we going to have?
We're going to have the hydrogen that got added and then we have this OH attaching itself here. We'd still have this methyl group here, and that carbonyl oxygen now becomes this negatively charged oxygen. And so, we have this structure here. Now, we're going to say the leaving group and intramolecular proton transfer are step 2.
Here we're going to say the C-O oxygen kicks out the phenoxide ion. And we're going to say that an intramolecular hydrogen ion transfer takes place as the phenoxide is kicked out. So what's going to happen here is we have this structure; oxygen uses its lone pairs to grab this hydrogen which causes this to be kicked to this oxygen. This happens because this oxygen decides to make a double bond here, which causes this movement to the oxygen here. So what I think we're going to get is, here's our benzene.
Here is our carboxylate anion that we have. We have this oxygen here, grabbing that hydrogen, so it's an OH, plus we have this carbon now becoming a carbonyl carbon again. It is connected to this methyl and it is connected to this OH. So this would give us our final products and how we got to them through steps 1 and step 2 of this whole process. Alright.
These are the steps you need to take, going from step 1 to step 2, to get to our final end results here.