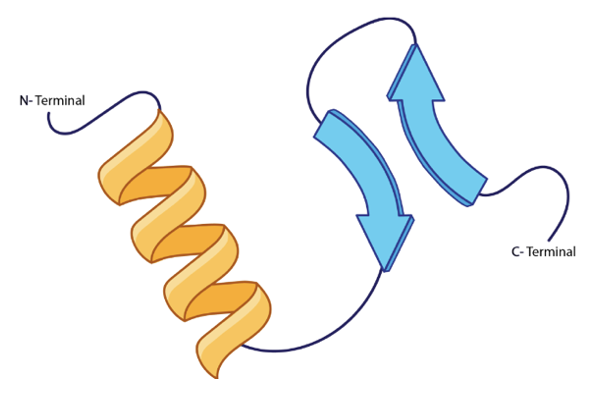
Now, when it comes to the secondary protein structure, we're going to say that this is the type of structure that results from hydrogen bonding of the atoms in the backbone of a protein. It involves the connection between the amide hydrogen of one peptide with the carbonyl oxygen of another. So if we take a look here at this image, we're going to say that this is our peptide chain 1, and this is our peptide chain 2. Here we have an amide, this NH group, because it's connected to a carbonyl. We just don't see it.
It's what's connected to it out of view. And this is our carbonyl oxygen. They will form a hydrogen bond with one another. Remember, hydrogen bonding is not actually bonding. It's an intermolecular force.
So we're going to show it and depict it through dashed bonds, so dashed lines. So that's one hydrogen bond. Here goes another amide nitrogen. Again, it's an amide because it's connected to this carbonyl here. So there goes the amide hydrogen, the carbon in the carbonyl oxygen.
So there goes another hydrogen bond. And here we just have two R groups. We don't specify what kind of R groups they are, so we don't know if hydrogen bonding would happen. So we don't do anything. So in this example, we have two sites where we see hydrogen bonding being used.
Alright. So just remember, it's this type of hydrogen bonding that exists, that happens, that helps to give us our secondary protein structure.