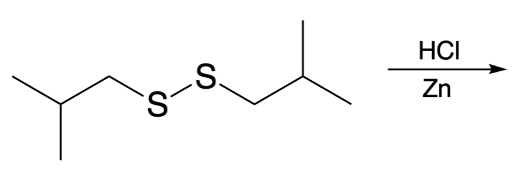
Hey, everyone. So in this example question, it says, illustrate the pentapeptide formed when a molecule of cysteine connected to glycine or dipeptide connects with cysteine, valine, and phenylalanine via a disulfide bridge. Right. So here we have a dipeptide, and here we have our tripeptide, and we need to connect them together. We know that the cysteines through their thiol groups are the ones that are going to connect to make the connection.
So if we're looking at it in just a simplistic way, we know that these two would be the ones connecting. Now, how exactly would we show this? Alright. So if we scroll down, we're going to draw here our remember, when we're drawing this peptide, we want to do the N-terminus end on the left and the C-terminus end on the right. Alright.
So, glycine is the simplest amino acid with its R group just being a hydrogen. So we're going to have here our protonated amino group. So here, I'm just doing the protonated form. So we have that. It's connected to our alpha carbon.
Our alpha carbon has its hydrogen and its R group is also another hydrogen. And here's our carbonyl. That carbonyl now is connected to the nitrogen group of our cysteine. So we're going to say here our cysteine we're drawing this connected to the alpha carbon. And it's connected to our alpha carbon.
That alpha carbon is connected to CH₂ group connected to an SH. And then here we'd have our carbonyl. Now, here's the thing. We're going to say at this point, this SH is going to connect to the other SH of the other thiol. They're connecting together to make this disulfide connection right here.
We're going to remove hydrogens from them. So here's our disulfide bridge. Now we just have to draw the rest of it. So again, we're going to have here, this connected to actually we're going to draw this in red to show the distinction. We're going to be connected to our CH₂, which is connected to our alpha carbon.
That alpha carbon there, it's going to have its amino group out here, then we're connected to this carbonyl, then we're connected so this is our cysteine, now we're going to do valine. Now we have our NH of valine. Valine's R group is an isopropyl. Then we have our carbonyl, then it's connected to the nitrogen of phenylalanine, and then that's connected to our alpha carbon, which is connected to a CH₂ and then a benzene ring. And then we have our carboxyl end here.
So this will represent our pentapeptide that we created and here is our disulfide bridge that connects the dipeptide to the tripeptide in order to make our pentapeptide.